In 2013, advancement in the mHSPC setting was demonstrated in SWOG 9346, concluding that continuous ADT therapy remains the standard; however, therapy should be tailored to a patient's individual needs with adequate counseling.1 The treatment for mHSPC has shifted since this time due to new clinical trial results.
Published trials to be mentioned include CHAARTED (docetaxel + ADT vs. ADT alone) 2, STAMPEDE (ADT + Abiraterone or ADT locally advanced and metastatic) 3, and LATITUDE, a phase 3 double-blind randomized trial of ADT with abiraterone plus prednisone or ADT alone in newly diagnosed high-risk metastatic hormone naïve PC patients 4.
The CHAARTED trial demonstrated significant overall survival (OS) benefit with a 13.6 months difference in favor of the docetaxel arm (median of 57.6 months vs. 44 months).
In the STAMPEDE trial, docetaxel and zoledronic acid were compared to the standard of care (ADT). A significant difference in OS was noted in favor of docetaxel (81 months vs. 71.3 months median OS). When looking at a subset analysis of metastatic patients only, a significant difference was noted in favor of the docetaxel arm (60 vs. 45 months median OS).
In LATTITUDE, patients were randomized to either ADT alone or ADT with abiraterone and prednisone 5. OS benefit was observed with the addition of abiraterone to ADT, demonstrating an HR of 0.63 (95% CI 0.52-0.76), p <0.0001, representing 37% improvement in OS against standard of care. When assessing the results of a subset analysis of this trial in metastatic patients only, the advantage of abiraterone was maintained with an HR of 0.61.
Lastly, in the LATITUDE trial, in the abiraterone + ADT arm, there was a 38% risk reduction of death, compared to ADT alone.
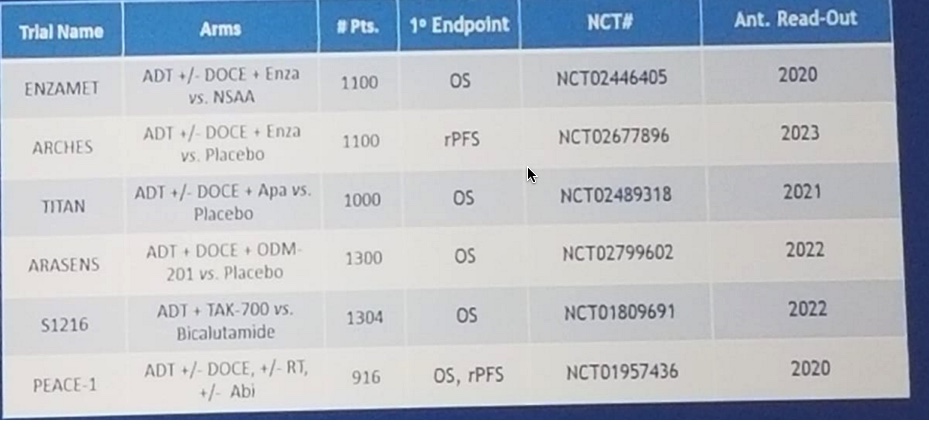
There are additional ongoing trials in the setting of mHSPC (Figure 1):
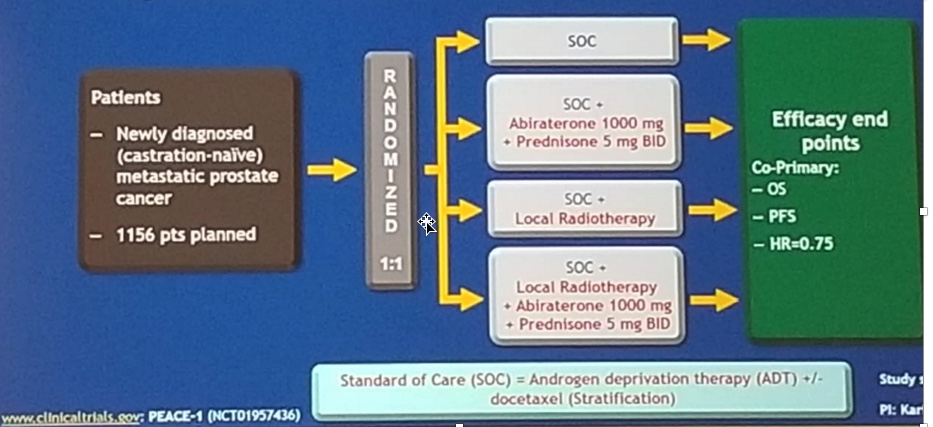
A question arises as to whether we should combine docetaxel and abiraterone together in this patient population. We hope to have this answered with the PEACE-1 randomized phase 3 trial. (Figure 2):
If the PEACE-1 trial results are positive, it may potentially carry with it enormous cost and toxicities. This will also beg the question, at what point is clinical benefit overshadowed by cost, practicality, and toxicities.
Agarwal went on to discuss the role of systemic therapy combined with definitive treatment of the primary tumor in mHSPC.
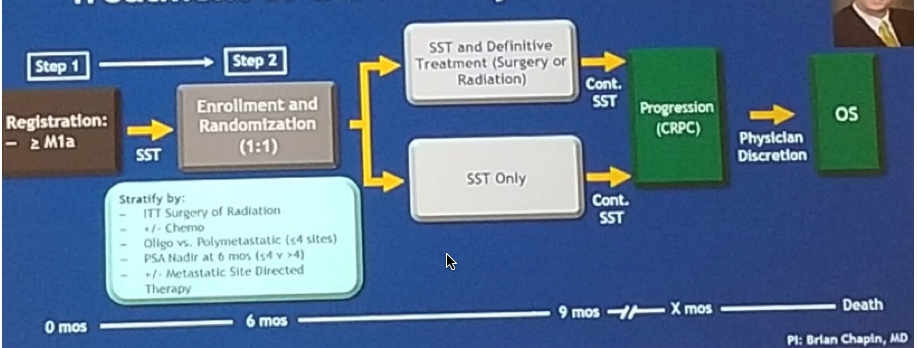
The SWOG 1802 trial is a randomized phase 3 trial of standard systemic therapy (SST) vs. SST + definitive treatment of the primary tumor (surgery to radiation) in mHSPC, led by Dr. Brian Chapin, from MD Anderson Cancer Center (trial design in figure 3):
This trial will potentially provide insights on the “cytoreductive treatment” of the primary tumor, in addition to systemic therapy in metastatic PC patients.
What is the implication for subsequent management in these patients? Currently, no prospective data exist only a few small retrospective studies. There is some data on sequencing from a retrospective follow-up of men on the GETUG AFU-15 trial 6 (ADT vs. ADT + docetaxel in mHSPC). In this retrospective trial 7, 1st line docetaxel, or abiraterone, or enzalutamide in MCRPC setting in patients from the GETUG AFU-15 trial were assessed. This analysis demonstrated a much better response for these 3 treatments in the MCRPC patients who were treated only with ADT in the mHSPC setting, than for those who received docetaxel in the MHSPC setting (Figure 4):

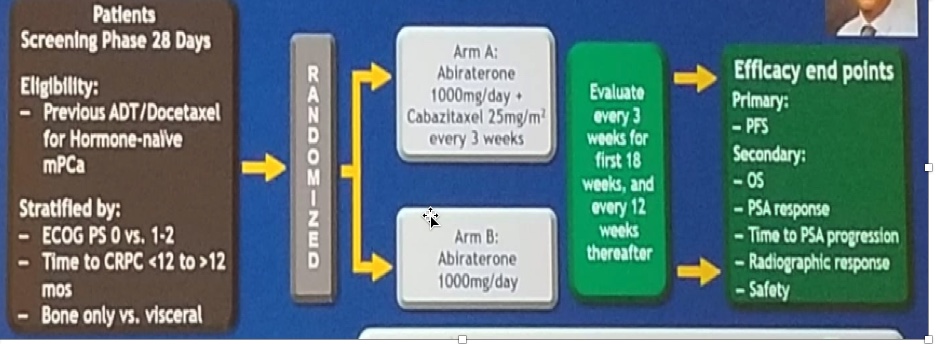
The CHAARTED 2 trial led by Dr. Christos E. Kyriakopoulos, from the University of Wisconsin, will assess abiraterone +/- Cabazitaxel for extensive disease following docetaxel (trial design in figure 5):
Agarwal concluded by stating that upfront treatment with either abiraterone or docetaxel is the new standard of care for men with mHSPC.
The data is, unfortunately, still not conclusive for the use of these agents in the M0 setting. In the setting of mHSPC, for low volume M1 disease, abiraterone should be given, while for high volume M1 disease, either abiraterone or docetaxel could be administered. No data exist to date to support adding abiraterone or docetaxel months after, for a patient already on ADT, or adding abiraterone after 6 cycles of docetaxel. Lastly, no recommendation regarding the sequencing of treatment can be made until we have biomarkers or prospective data.
Presented by: Neeraj Agarwal, MD, University of Utah, Salt Lake City, UT, USA
Written By: Hanan Goldberg, MD, Urologic Oncology Fellow (SUO), University at the 2018 ASCO Annual Meeting June 1-5 -Chicago, IL
References:
1. Hussain M. et al. Intermittent versus Continuous Androgen Deprivation in Prostate Cancer N Eng J Med 2013; 368:1314-25
2. Sweeney CJ et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer. N Engl J Med. 2015 Aug 20;373(8):737-46. doi: 10.1056/NEJMoa1503747. Epub 2015 Aug 5.
3. James ND. et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet 2016; 387:1163-77
4. Fizazi K. et al. Abiraterone plus Prednisone in Metastatic, Castration-Sensitive Prostate Cancer. N Engl J Med. 2017 Jul 27;377(4):352-360. doi: 10.1056/NEJMoa1704174. Epub 2017 Jun 4.
5. James ND. et al. Abiraterone for Prostate Cancer Not Previously Treated with Hormone Therapy. N Eng J. Med 2017; 377:338-351
6. Gravis G et al. Androgen-deprivation therapy alone or with docetaxel in non-castrate metastatic prostate cancer (GETUG-AFU 15): a randomized, open-label, phase 3 trial. Lancet Oncol. 2013 Feb;14(2):149-58. doi: 10.1016/S1470-2045(12)70560-0. Epub 2013 Jan 8.
7. Lavaud P. et al. Anticancer Activity and Tolerance of Treatments Received Beyond Progression in Men Treated Upfront with Androgen Deprivation Therapy With or Without Docetaxel for Metastatic Castration-naïve Prostate Cancer in the GETUG-AFU 15 Phase 3 Trial. Eur Urol 2018, 73(5): 696-703
In the STAMPEDE trial, docetaxel and zoledronic acid were compared to the standard of care (ADT). A significant difference in OS was noted in favor of docetaxel (81 months vs. 71.3 months median OS). When looking at a subset analysis of metastatic patients only, a significant difference was noted in favor of the docetaxel arm (60 vs. 45 months median OS).
In LATTITUDE, patients were randomized to either ADT alone or ADT with abiraterone and prednisone 5. OS benefit was observed with the addition of abiraterone to ADT, demonstrating an HR of 0.63 (95% CI 0.52-0.76), p <0.0001, representing 37% improvement in OS against standard of care. When assessing the results of a subset analysis of this trial in metastatic patients only, the advantage of abiraterone was maintained with an HR of 0.61.
Lastly, in the LATITUDE trial, in the abiraterone + ADT arm, there was a 38% risk reduction of death, compared to ADT alone.
There are additional ongoing trials in the setting of mHSPC (Figure 1):

A question arises as to whether we should combine docetaxel and abiraterone together in this patient population. We hope to have this answered with the PEACE-1 randomized phase 3 trial. (Figure 2):

If the PEACE-1 trial results are positive, it may potentially carry with it enormous cost and toxicities. This will also beg the question, at what point is clinical benefit overshadowed by cost, practicality, and toxicities.
Agarwal went on to discuss the role of systemic therapy combined with definitive treatment of the primary tumor in mHSPC.
The SWOG 1802 trial is a randomized phase 3 trial of standard systemic therapy (SST) vs. SST + definitive treatment of the primary tumor (surgery to radiation) in mHSPC, led by Dr. Brian Chapin, from MD Anderson Cancer Center (trial design in figure 3):

This trial will potentially provide insights on the “cytoreductive treatment” of the primary tumor, in addition to systemic therapy in metastatic PC patients.
What is the implication for subsequent management in these patients? Currently, no prospective data exist only a few small retrospective studies. There is some data on sequencing from a retrospective follow-up of men on the GETUG AFU-15 trial 6 (ADT vs. ADT + docetaxel in mHSPC). In this retrospective trial 7, 1st line docetaxel, or abiraterone, or enzalutamide in MCRPC setting in patients from the GETUG AFU-15 trial were assessed. This analysis demonstrated a much better response for these 3 treatments in the MCRPC patients who were treated only with ADT in the mHSPC setting, than for those who received docetaxel in the MHSPC setting (Figure 4):

The CHAARTED 2 trial led by Dr. Christos E. Kyriakopoulos, from the University of Wisconsin, will assess abiraterone +/- Cabazitaxel for extensive disease following docetaxel (trial design in figure 5):

Agarwal concluded by stating that upfront treatment with either abiraterone or docetaxel is the new standard of care for men with mHSPC.
The data is, unfortunately, still not conclusive for the use of these agents in the M0 setting. In the setting of mHSPC, for low volume M1 disease, abiraterone should be given, while for high volume M1 disease, either abiraterone or docetaxel could be administered. No data exist to date to support adding abiraterone or docetaxel months after, for a patient already on ADT, or adding abiraterone after 6 cycles of docetaxel. Lastly, no recommendation regarding the sequencing of treatment can be made until we have biomarkers or prospective data.
Presented by: Neeraj Agarwal, MD, University of Utah, Salt Lake City, UT, USA
Written By: Hanan Goldberg, MD, Urologic Oncology Fellow (SUO), University at the 2018 ASCO Annual Meeting June 1-5 -Chicago, IL
References:
1. Hussain M. et al. Intermittent versus Continuous Androgen Deprivation in Prostate Cancer N Eng J Med 2013; 368:1314-25
2. Sweeney CJ et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer. N Engl J Med. 2015 Aug 20;373(8):737-46. doi: 10.1056/NEJMoa1503747. Epub 2015 Aug 5.
3. James ND. et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet 2016; 387:1163-77
4. Fizazi K. et al. Abiraterone plus Prednisone in Metastatic, Castration-Sensitive Prostate Cancer. N Engl J Med. 2017 Jul 27;377(4):352-360. doi: 10.1056/NEJMoa1704174. Epub 2017 Jun 4.
5. James ND. et al. Abiraterone for Prostate Cancer Not Previously Treated with Hormone Therapy. N Eng J. Med 2017; 377:338-351
6. Gravis G et al. Androgen-deprivation therapy alone or with docetaxel in non-castrate metastatic prostate cancer (GETUG-AFU 15): a randomized, open-label, phase 3 trial. Lancet Oncol. 2013 Feb;14(2):149-58. doi: 10.1016/S1470-2045(12)70560-0. Epub 2013 Jan 8.
7. Lavaud P. et al. Anticancer Activity and Tolerance of Treatments Received Beyond Progression in Men Treated Upfront with Androgen Deprivation Therapy With or Without Docetaxel for Metastatic Castration-naïve Prostate Cancer in the GETUG-AFU 15 Phase 3 Trial. Eur Urol 2018, 73(5): 696-703


