Figure 1: the different response to ADT in HSPC and CRPC:

An important question that clinicians may ask themselves, is whether earlier more intense ADT will result in higher cure rates in localized high-risk prostate cancer (PC) or oligo-metastatic PC. There are several trials in different development stages assessing 2nd generation ADT in high risk localized HSPC. These include assessing the effect of radiotherapy with abiraterone, enzalutamide, and apalutamide (D’Amico A, in development). Another trial is assessing neoadjuvant treatment with abiraterone, enzalutamide, and apalutamide before radical prostatectomy (Taplin ME et al., phase 3 trial in development). Lastly, there is a Phase II, Randomized, 2-Arm Study of ADT+ Apalutamide and abiraterone vs. ADT + Apalutamide in high risk localized, or low volume metastatic HSPC. This is called the METACURE trial (NCT03436654).
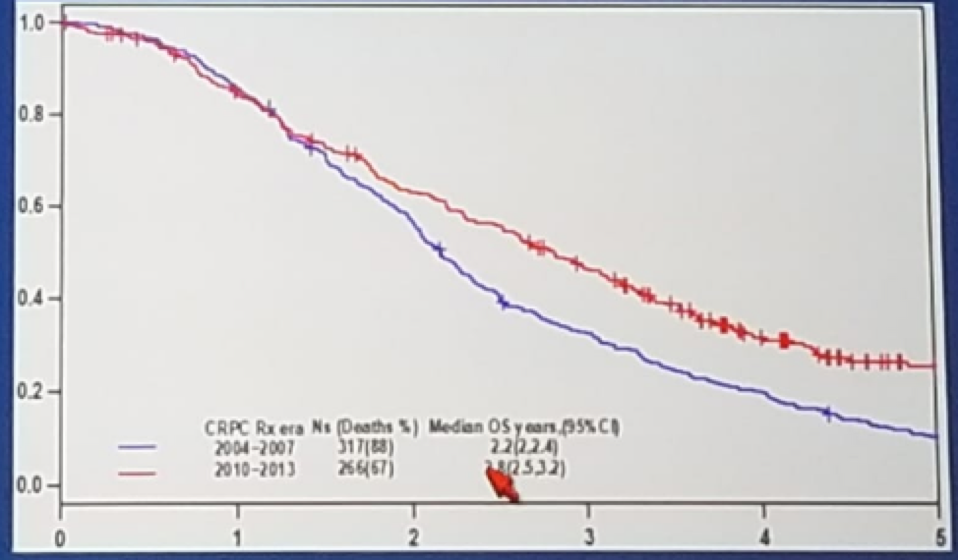
Another important question to be answered is how do we select new targets and combinations of therapies that will delay progression and improve survival. In a nice study [1], the impact of new systemic therapies on OS of patients with metastatic CRPC was analyzed. This demonstrated that therapies approved since 2010 showed a modest impact in aggregate on the median OS in MCRPC patients, with a median improvement of only 7 months, (Figure 2).
Figure 2: Kaplan-Meyer of overall survival estimates according to the use of new drug therapies for MCRPC patients in the overall population:
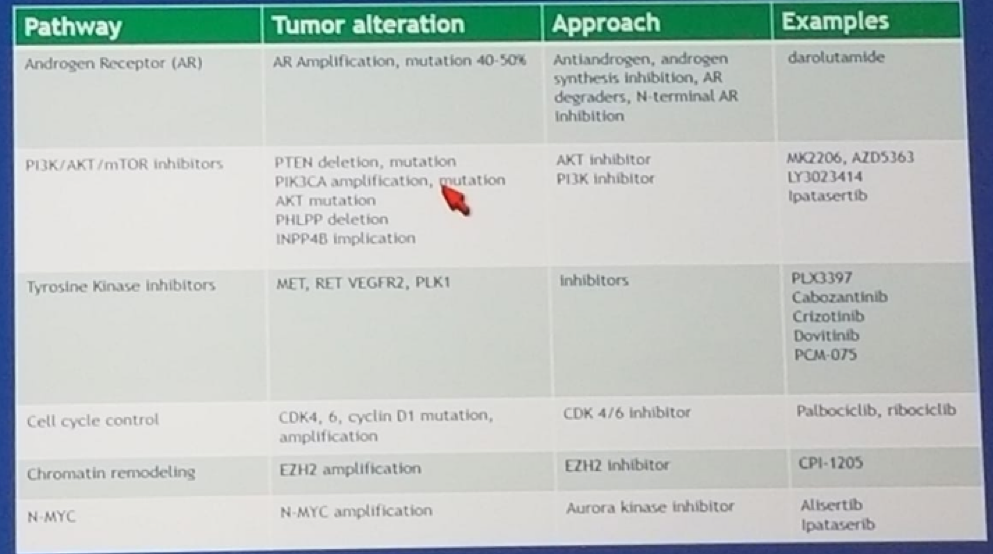
Circulating tumor cells, and circulating DNA are more commonly used today in cancer care. This allows us to identify multiple genomic abnormalities, which include: point mutations, copy number changes, translocations, and epigenetic changes. Additionally, there are currently several phase 1 / 2 trials of signaling pathway inhibition in MCRPC patients (Figure 3).
Figure 3: Selected phase 1 / 2 trials of signaling pathway inhibitors in mCRPC:
In summary, next-generation AR therapies have the potential to improve cure rates in early stage, high risk, HSPC. In MCRPC in aggregate therapies approved since 2010 have improved survival only modestly. The genomics of MCRPC is complicated and heterogeneous. Biomarkers are needed to direct therapies in the future. Finally, many new targets and combinations approaches are in preclinical and early clinical development and hold promise.
References:
1. Edoardo Francini, et al. Impact of new systemic therapies on overall survival (OS) of patients (pts) with metastatic castration resistant prostate cancer (mCRPC) in a hospital-based registry. 10.1200/JCO.2018.36.6_suppl.203 Journal of Clinical Oncology 36, no. 6_suppl (February 20 2018) 203-203.
Presented by: Mary Ellen Taplin, Harvard Medical School, Boston, MA, USA
Written By: Hanan Goldberg, MD, Urologic Oncology Fellow (SUO), University of Toronto, Princess Margaret Cancer Centre, Twitter: @GoldbergHanan at the 2018 ASCO Annual Meeting - June 1-5, 2018 – Chicago, IL USA


