Over a median follow-up of 30.4 months, patients treated with ADT + AA + prednisone had a 38% risk reduction of death (HR 0.62, 95%CI 0.51-0.76) compared to ADT + placebo. Median OS was not yet reached in the ADT + AA + prednisone arm compared to 34.7 months in the ADT + placebo arm. OS rates at 3 years for the ADT + AA + prednisone arm was 66%, compared to 49% in the ADT + placebo arm. There was also 53% risk of reduction of radiographic progression or death for patients treated with ADT + AA + prednisone (median 33.0 months; HR 0.47, 95%CI 0.39-0.55) compared to ADT + placebo (14.8 months).
Third, there was statistically significant improvement across all secondary endpoints for ADT + AA + prednisone: (i) time to PSA progression (HR 0.30, 95%CI 0.26-0.35), (ii) time to pain progression (HR 0.70, 95%CI 0.58-0.83), (iii) time to next symptomatic skeletal event (HR 0.70, 95%CI 0.54-0.92), (iv) time to chemotherapy (HR 0.44, 95%CI 0.35-0.56), and (v) and time to subsequent prostate cancer therapy (HR 0.42, 95%CI 0.35-0.50). In addition to these oncologic outcomes, median time to deterioration of functional status assessed by the FACT-P total score scale was 12.9 months (95%CI 9.0-16.6) for patients receiving ADT + AA + prednisone, as compared to 8.3 months (7.4-11.1) in the ADT + placebo group (HR 0.85, 95%CI 0.74-0.99) [2]. Based on the above findings, the study was unblinded at the time of the first interim analysis. At ASCO 2018 annual meeting, Dr. Fizazi and colleagues presented longer term efficacy and safety analyses from the phase III LATITUDE trial.
Patients enrolled in LATITUDE had to be newly diagnosed with prostate cancer and be high-risk, defined as meeting at least two of three criteria: (i) Gleason score ≥8, (ii) presence of ≥3 lesions on bone scan, (iii) presence of measurable visceral lesions. Patients were stratified by the presence of visceral disease (yes/no) and ECOG performance status (0, 1 vs 2) and then randomized 1:1 to either ADT + AA (1000 mg daily) + prednisone (5 mg) (n=597) or ADT + placebo (n=602). For this analysis (from the preplanned second interim analysis at ~554 deaths), a stratified proportional hazards model assessed longer term follow-up on OS, secondary end points, and adverse events (AEs), including those associated with mineralocorticoid excess.
At the time of this analysis, the median follow-up was 41.0 months (range 0.1-54.0), 10.6 months longer than the initial analysis. There were 205 patients (34%) in the ADT + AA + prednisone arm and 70 patients (12%) in the ADT + placebo arm (of whom 57 patients (81%) had crossed over to ADT + AA + prednisone) who remained on treatment. Updated OS results continued to favor ADT + AA + prednisone (NR vs 36.7 months; HR 0.638, 95%CI 0.538-0.758):
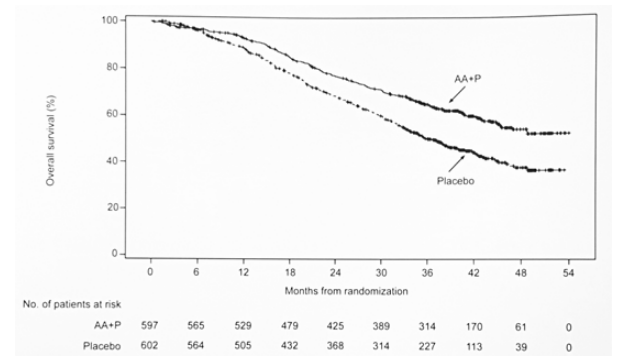
The results of other secondary end points:
- Time to pain progression: 47.4 vs. 17. 9 months; HR 0.723, 95%CI 0.608-0.860
- Time to skeletal-related event: NR vs NR; HR 0.739, 95%CI 0.579-0.942
- Time to chemotherapy initiation: NR vs 47.3 months; HR 0.471, 95%CI 0.378-0.586
- Time to subsequent prostate cancer therapy: NR vs 21.2 months; HR 0.428, 95%CI 0.361-0.507
The impressive outcomes of LATITUDE continue to demonstrate a 36% reduction in risk of death among patients receiving ADT + AA + prednisone, despite most patients receiving ADT + placebo crossing over to receive ADT + AA + prednisone. Dr. Fizazi concluded that given this durable reduction in risk of death, coupled with secondary endpoints continuing to favor the ADT + AA + prednisone arm, AA + prednisone should unequivocally remain the standard of care for patients with newly diagnosed, high-risk, hormone-naïve prostate cancer.
Presented By: Karim Fizazi, Gustave Roussy, University of Paris Sud, Villejuif, France
Co-Authors: Susan Feyerabend, Nobuaki Matsubara, Mustafa Ozguroglu, Luis Enrique Fein, Alfredo Rodríguez Antolín, Boris Yakovlevich Alekseev, Giri Sulur, Andrew Protheroe, Peter De Porre, Susan Li, Youn C. Park, Suneel Mundle, Namphuong Tran, Kim N. Chi; Studienpraxis Urologie, Nürtingen, Germany; National Cancer Center Hospital East, Chiba, Japan; Cerrahpaşa Medical Faculty, Istanbul University, Istanbul, Turkey; Instituto de Oncología de Rosário, Rosário, Argentina; Hospital Universitario 12 de Octubre, Madrid, Spain; P.A. Hertsen Moscow Cancer Research Institute, Moscow, Russian Federation; Janssen Research & Development, Los Angeles, CA; Oxford University Hospitals Foundation NHS Trust, Oxford, United Kingdom; Janssen Research & Development, Beerse, Belgium; Janssen Research & Development, Spring House, PA; Janssen Research & Development, Raritan, NJ; BC Cancer Agency - Vancouver Centre, Vancouver, BC, Canada
Written by: Zachary Klaassen, MD, Urologic Oncology Fellow, University of Toronto, Princess Margaret Cancer Centre, Twitter: @zklaassen_md at the 2018 ASCO Annual Meeting - June 1-5, 2018 – Chicago, IL USA
References:
1. Fizazi K, Tran N, Fein L, et al. Abiraterone plus Prednisone in Metastatic, Castration-Sensitive Prostate Cancer. N Engl J Med. 2017;377(4):352-360.
2. Chi KN, Protheroe A, Rodriguez-Antolin A, et al. Patient-reported outcomes following abiraterone acetate plus prednisone added to androgen deprivation therapy in patients with newly diagnosed metastatic castration-naïve prostate cancer (LATITUDE): An international, randomized phase 3 trial. Lancet Oncol. 2018;19(2):194-206.


