It should be noted that the burden for proof is much higher for these patients as current treatment options are generally well tolerated and have high cure rates. As such, at this time, studies in this population are limited to patients with refractory disease who have failed all other options.
In this study, entitled APACHE, durvalumab (anti-PD-1, Astra-Zeneca) was used alone or in combination with tremelimumab (anti-CTLA-4, MedImmune/Astra-Zeneca), in patients with advanced germ cell tumors – this was an open-label phase II randomized study. The authors report the end of the first stage of the study.
As mentioned before, these are patients with few remaining options. The prognosis of chemorefractory GCT is dismal. Only men who have failed ≥2 chemotherapy (CT) regimens were eligible for the study.
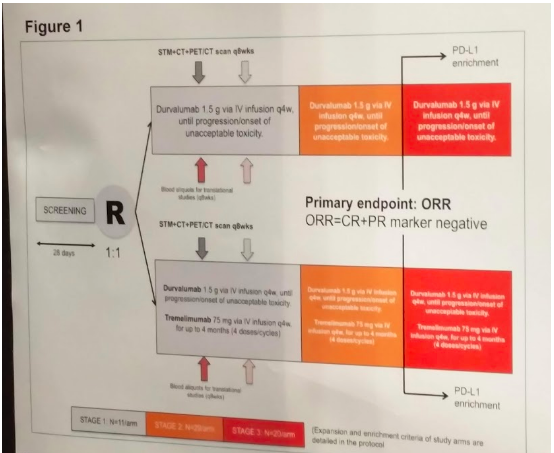
They were randomized to receive either Durva, 1.5 gr q4w, x 13 cycles (arm A) or Durva plus with Treme, 75 mg q4w, x 4 cycles, followed by Durva alone x total 13 cycles (arm B). Serum tumor markers (STM), and radiologic assessments are repeated q8 weeks. Biomarker analyses include: IHC PD-L1 expression on immune cells (Ventana SP142) and genomic sequencing with FoundationOne assay (Foundation Medicine Inc., Cambridge, MA, USA) as additional analyses.
The primary endpoint is the modified objective response-rate (mORR). Per the RECIST 1.1 criteria, the mORR includes complete (CR) or partial response [PR] or stable disease [SD]+STM reduction > 10%. The null hypothesis was that mORR would be less than 10%; a positive study was defined as a mORR ≥25%.
This was a staged study. The total sample size of 120 pts was split into 3 stages: in stage 1, according to Gehan’s rule, each arm is terminated whenever no response is observed. Results of first stage are presented today. Based on the first stage, a decision will be made to purse either arm into a 2nd stage.
Full protocol below:
Over a 9 month period (2/2017 to 11/2018), 18 patients were enrolled (17 male, 1 female?), 9 per each arm of the study. Median age 33. Of these 18 patients, 14 had gonadal and 4 extragonadal GCT. More importantly, 15 had received ≥3 prior CT regimens – demonstrating severe CT resistance and limited alternative options. Median tumor marker levels at baseline: 5,105 AFP and 6519 HCG. Most (16 of 18) were NSGCT or mixed histology.
In terms of genomic analysis. median tumor mutational burden (TMB) was 4 mutations (mut)/mb.
In terms of adverse events and tolerability, only 1 patient (5.6%) had a reversible grade 3 immune-related AE (pneumonitis) in arm B. Otherwise, there were no other Grade 3-5 adverse events, so they were well tolerated.
In terms of clinical outcomes, outcomes were mixed. In arm A, 100% had disease progression (PD), all with features of hyperprogression (hyper-PD): 4 had clinical PD and death before restaging, median increase in sum of tumor diameters (RECIST 1.1) was 146%, median increase in the elevated STM was 462%. In contrast, in arm B, 2 responses (22.2%, 1 RECIST-PR in seminoma and 1 SD with STM reduction in nonseminoma) were observed. However, in the patients who progressed, PD features were similar to arm A. PD occurred in both arms regardless of PD-L1 expression and TMB (PR case: PD-L1 negative and 4 mut/mb).
Median follow-up was 5.1 months. Median PFS 1.4 months and median OS 2.9 months.
Based on this, the authors note that single-agent durvalumab should not and will not be pursued further in GCT. However, based on the 2 person response in arm B, they feel that combination immunotherapy showed signals of activity in a small subset of patients and will be expanded to 2nd stage evaluation. At this time, we don’t have predictors to identify these patients – unfortunately, non-responders may show signs of hyperprogression.
Presented By:Daniele Raggi
Co-Authors: Patrizia Giannatempo, Luigi Mariani, Maurizio Colecchia, Giuseppina Calareso, Roberto Salvioni, Siraj Mahamed Ali, Jeffrey S. Ross, Jon Chung, Andrea Necchi
Institution(s):Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy; Radiology Unit, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy; Foundation Medicine, Inc., Cambridge, MA; SUNY Upstate Medical University, Syracuse, NY; Istituto Nazionale dei Tumori, Milan, Italy
Written by: Thenappan Chandrasekar, MD, Clinical Fellow, University of Toronto, Twitter: @tchandra_uromd at the 2018 ASCO Annual Meeting - June 1-5, 2018 – Chicago, IL USA


