(UroToday.com) The 2024 Advanced Prostate Cancer Consensus Conference (APCCC) meeting featured a session on PSMA for diagnostics and treatment, and a presentation by Dr. Michael Hofman discussing the interchangeability of alternative PSMA ligands for diagnostics and treatment. Dr. Hofman started by highlighting that there are currently four common radiotracers: 68Ga-PSMA-11, 18F-DCFPyL, 18F-PSMArh7.3, and 18F-PSMA-1007, with the aforementioned first three being FDA-approved. According to Dr. Hofman, 68Ga-PSMA-11 and 18F-DCFPyL are equivalent and interchangeable, with normal and similar organ biodistribution (intra-individual comparison):

Notably, amongst the four radiotracers, there are differences in biodistribution: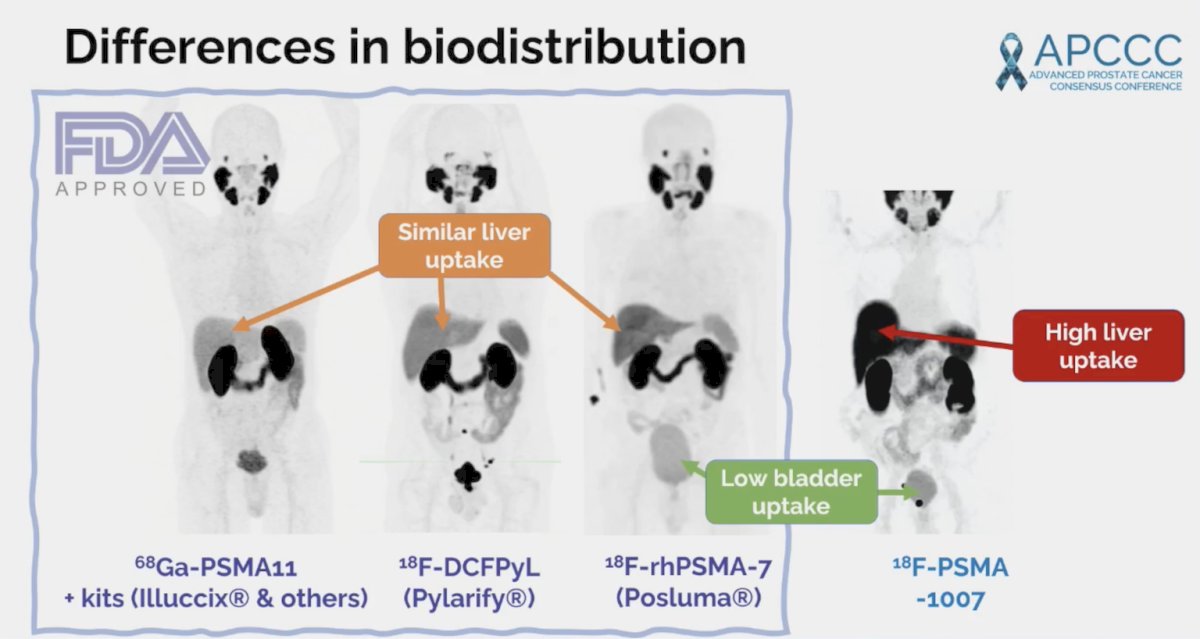
In Australia, more than 10,000 studies are being performed per year and reimbursement is agnostic, thus allowing the physician to choose the radiotracer they desire. Of note, there may be important differences between PET scanners whether it is digital or an analog: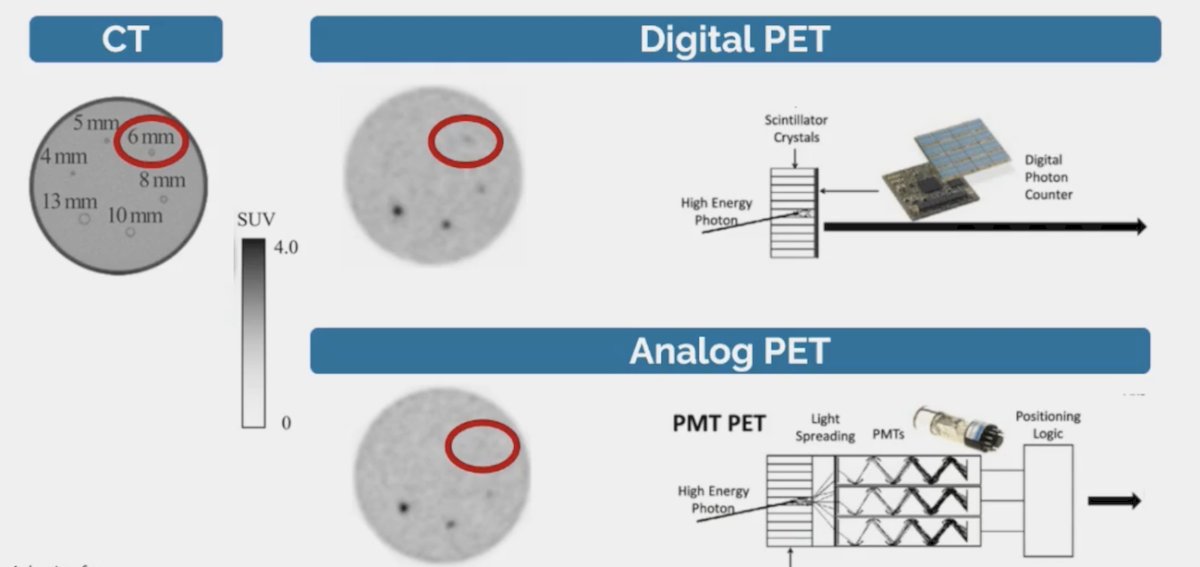
Indeed, technological advances are outpacing “evidence base”, and even for bone scans we have two agents (MDP versus HDP):
Are there radioligand-specific differences in tumor uptake? A comparison of the UCSF/UCLA 68Ga-PSMA-11 study,1 CONDOR trial for 18F-DCFPyL,2 and SPOTLIGHT trial for 18F-PSMArh7.33 shows a PPV of 92% in the UCLA/UCSF trial, a correct localization rate of 85%-87% in CONDOR, and a combined region-level PPV of 64% in SPOTLIGHT: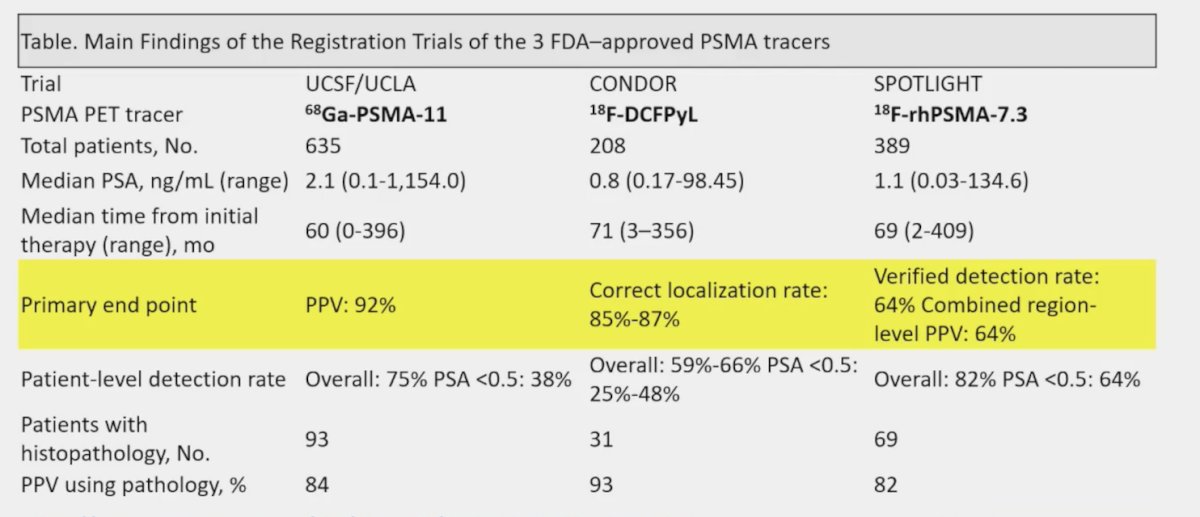
However, Dr. Hofman notes that there are several issues:
- There are few comparative studies of the different ligands
- “PSMA PET-new” is better than CT/bone scan, but is it better than 68Ga-PSMA-11 or 18F-DCFPyL?
- The era of using CT and bone scan as the comparator (“gold standard”) must end
Regarding 18F-PSMA-1007, we must be aware of benign bone uptake: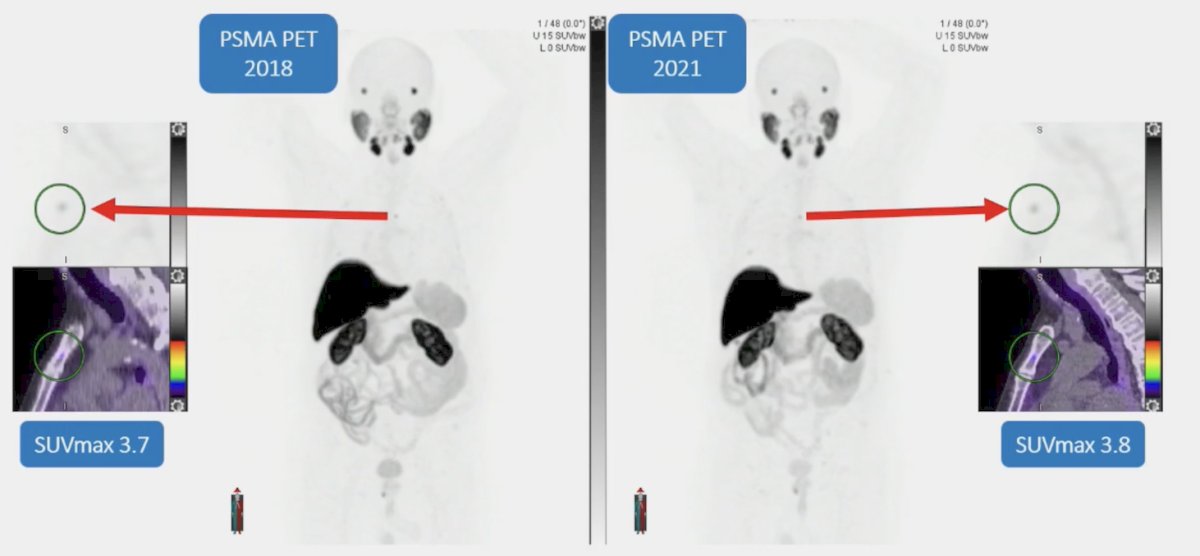
Dr. Hofman summarized bone uptake with the following points:
- 18F dissociation from PSMA-1007 leads to benign bone uptake, which is not described for 18F-DCFPyL or 18F-PSMArh7.3
- 48% of PSMA-1007 versus 15% of PSMA-11 may have benign bone uptake
- SUVmax: 6.2 vs 2.4
- Lesions being called benign bone decrease with reader experience
- This is a challenging problem, especially for staging or oligometastatic disease
For PSMA radioligand therapy, PSMA-11, PSMA-617 and PSMA-I&T all have the same binding motif, but different chelator: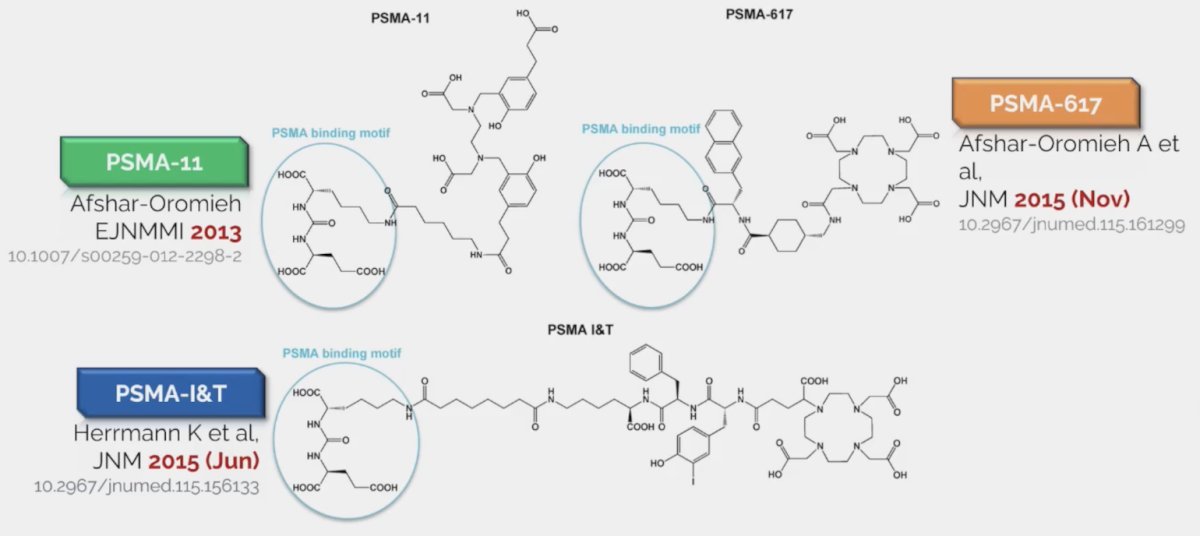
However, are PSMA-617 and PSMA-I&T equivalent? Dr. Hofman notes that there is much more data for PSMA-617 as compared to PSMA-I&T: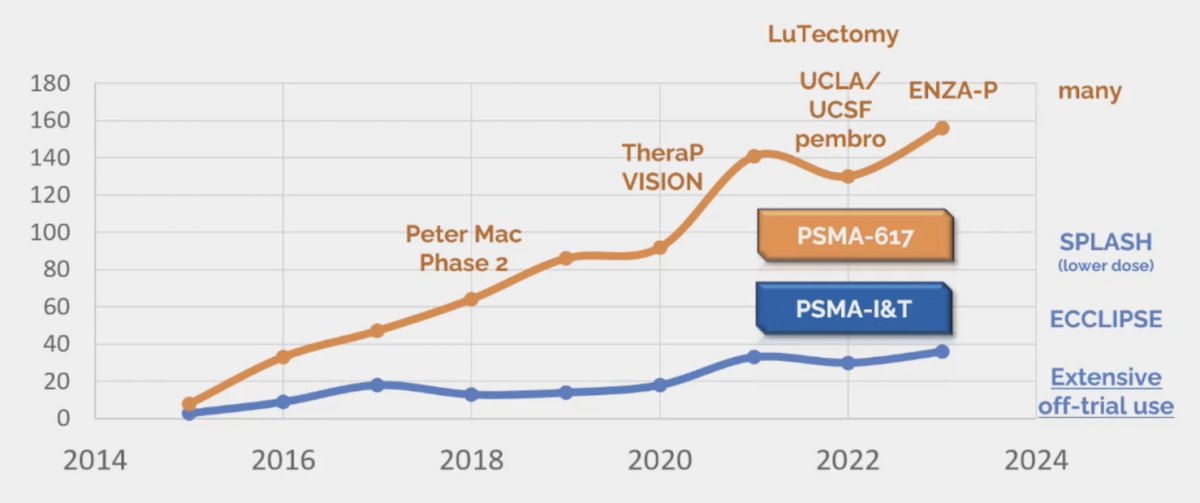
Sadaghiani et al.4 performed a systematic review and meta-analysis highlighting data the use of Lu-177 in patients with mCRPC. The authors included a total of 24 studies with 1,192 patients who had received Lu-177 based therapy. The majority of these studies reported on 177Lu-PSMA-617 (20 studies) while 3 reported on 177Lu-PSMA-I&T, and 1 reported on both. In this heavily pre-treated population, 44% of patients (95% CI 39-50%) had a PSA response of at least 50% among those receiving 177Lu-PSMA-617 with similar, though slightly lower numbers among those receiving 177Lu-PSMA-I&T (36%, 95% CI 26-47%). Further, these authors found that treatment was well tolerated with very low rates of grade 3 or 4 toxicity, with the highest observed rates for anemia at 8% (95% CI 5-12%). Based on these results MSAC (an independent Australian health technology assessment committee) termed these two agents “radioequivalent.” Despite the limitations in the evidence comparing products, MSAC accepted that these two products are mutually noninferior for patient outcomes.
Regarding 177Lu-PSMA, is it a drug or a type of radiotherapy?
- Arguments for the radiotherapy model:
- Uses dosimetry to define dose to critical organs
- We cannot exceed the known maximum tolerated limits to critical organs
- Toxicities are delayed: short term follow-up is inadequate
- Its limits are theoretical and are extrapolated from external-beam radiotherapy
- Arguments for the drug model:
- There is no need for dosimetry or post-treatment imaging
- Maximum tolerated dose is defined with phase 1 dose escalation studies
- Acute toxicities are observed and definable
- There is a predictable delayed cumulative toxicity that may be missed
A 2022 study from Schuchardt et al.5 compared the safety, biodistribution, and dosimetry for 177Lu-PSMA I&T and 177Lu-PSMA-617 among 138 patients undergoing radioligand therapy for mCRPC. This study found a lower renal dose with PSMA-617 (0.77 vs 0.92 Gy/GBq, p = 0.0015), but with comparable tumor doses and larger interpatient variability: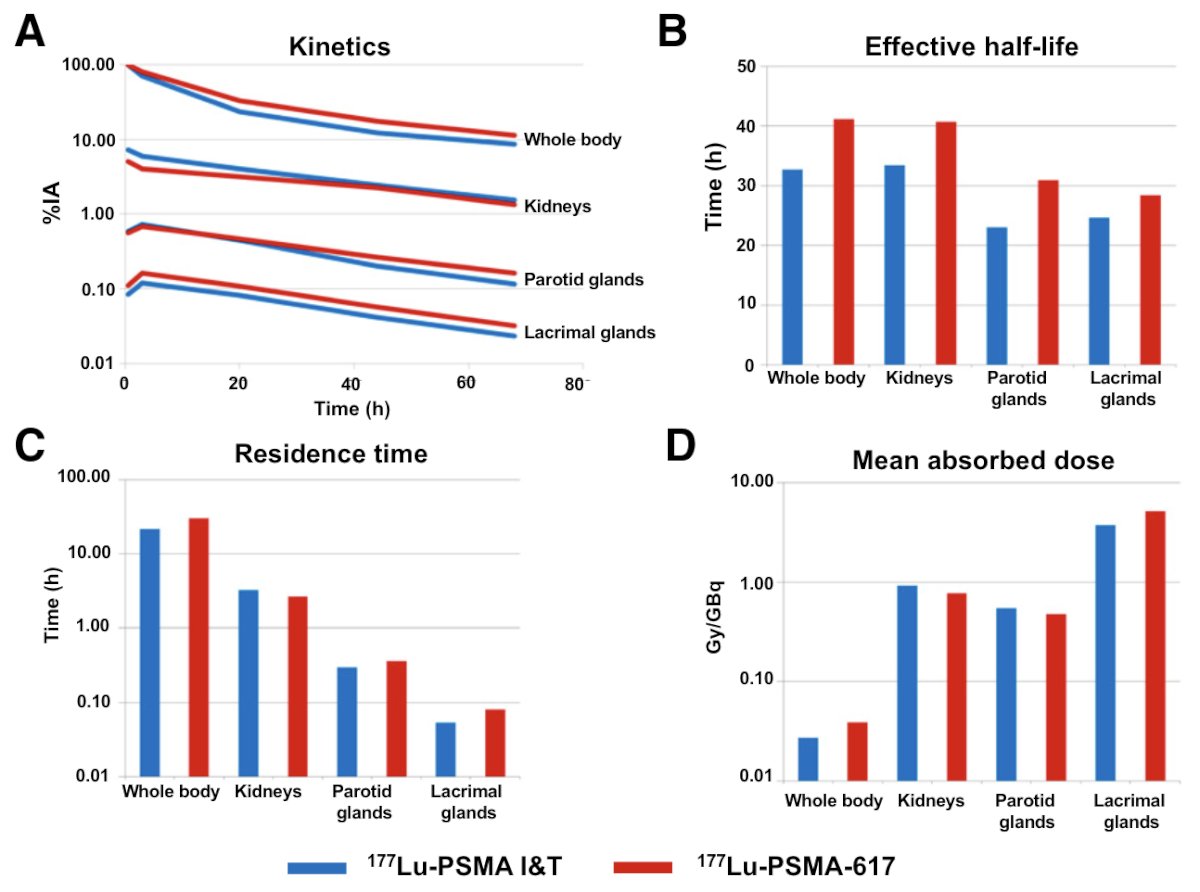
At the 2022 APCCC meeting, the question “can the data generated by the VISION trial with PSMA-617 be extrapolated to PSMA-I&T”? at which time 68% of respondents said no, it could not. However, a recent survey of 95 theranostic centers suggested that 27% were using both agents, 51% were using only PSMA-617, and 22% were using only PSMA-I&T.
Importantly, Dr. Hofman notes that for precision medicine, patient selection is key. Specifically, FDG PET/CT makes invisible PSMA-negative bone lesions visible, thus we should not be treating blindly. Moreover, new ligands must be compared to PSMA-617 or PSMA-I&T. For now, it appears the small molecule peptides have won versus the antibodies: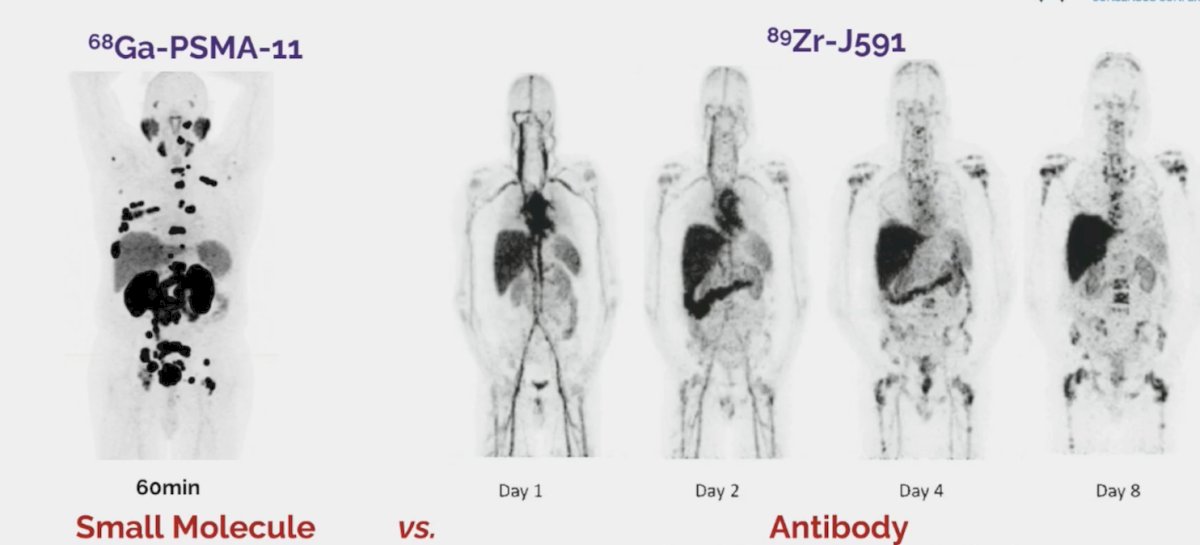
Compared to the antibodies, small molecules (i) have higher uptake and better tumor dosimetry, (ii) have rapid clearance and lower toxicity, (iii) are cheaper, however (iv) when it comes to the alpha emitters, this may be different.
Dr. Hofman concluded his presentation discussing the interchangeability of alternative PSMA ligands for diagnostics and treatment with the following take-home messages:
- The best PSMA radiotracer for PET/CT is the one you can access
- 68Ga-PSMA-11 and 18F-DCFPyL are essentially equivalent
- 18F-PSMArh7.3 is the newest FDA approved radiotracer, but there are questions as to whether it is equivalent and providers have far less experience with it
- For 18F-PSMA-1007, we must be aware of benign bone uptake
- PSMA-617 and PSMA-I&T seem to be radio-equivalent
- The “peptides” win, for the moment
- Reader experience, patient selection, and technical factors all matter
Presented by: Professor Michael Hofman, MBBS, FRACP, FAANMS, FICIS, GAICD, Prostate Cancer Theranostics and Imaging Centre of Excellence, Peter MacCallum Cancer Center, Melbourne, Australia
Written by: Zachary Klaassen, MD, MSc - Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 Advanced Prostate Cancer Consensus Conference (APCCC) Meeting, Lugano, Switzerland, Thurs, Apr 25 - Sat, Apr 27, 2024.
References:
- Calais J, Czernin J, Cao M, et al. 68GA-PSMA-11 PET/CT mapping of prostate cancer biochemical recurrence after radical prostatectomy in 270 patients with a PSA level of less than 1.0 ng/mL: Impact on salvage radiotherapy planning. J Nucl Med 2018 Feb;59(2):230-237.
- Morris MJ, Rowe SP, Gorin MA, et al. Diagnostic Performance of 18F-DCFPyL-PET/CT in Men with Biochemically Recurrent Prostate Cancer: Results from the CONDOR Phase III, Multicenter Study. Clin Cancer Res. 2021 Jul 1;27(13):3674-3682.
- Jani AB, Ravizzini GC, Gartrell BA, et al. Diagnostic performance and safety of 18F-rhPSMA-7.3 positron emission tomography in men with suspected prostate cancer recurrence: Results from a phase 3, prospective, multicenter study (SPOTLIGHT). J Urol. 2023 Aug;210(2):299-311.
- Sadaghiani MS, Sheikhbahaei S, Werner RA, et al. A systematic review and meta-analysis of the effectiveness and toxicities of Lutetium-177-labeled Prostate-specific membrane antigen-targeted radioligand therapy in metastatic castration-resistant prostate cancer. Eur Urol. 2021 Jul;80(1):82-94.
- Schuchardt C, Zhang J, Kulkarni HR, et al. Prostate-Specific Membrane Antigen Radioligand Therapy using 177Lu-PSMA I&T and 177Lu-PSMA-617 in Patients with Metastatic Castration-Resistant Prostate Cancer: Comparison of Safety, Biodistribution, and Dosimetry. J Nucl Med. 2022 Aug;63(8):1199-1207.


