(UroToday.com) The 2024 Advanced Prostate Cancer Consensus Conference (APCCC) meeting featured a session on the management of mHSPC, and a presentation by Dr. Bertrand Tombal discussing the patients with mHSPC that can be offered a treatment break and how to manage this break. The current standard of care for treatment mHSPC is the association of ADT and one of the available ARTA, +/- docetaxel and/or prostate radiotherapy. This treatment intensification increases overall survival by ~30%, as well as other oncological endpoints, although with an increase in cost and toxicity:
- Increased risk of cardiovascular events (RR 1.71, 95% CI 1.29-2.27) and grade 3-4 HTA (RR 1.53, 95% CI 1.19-1.97)
- Increased risk of falls and fractures and falls
- Increased risk of cognitive toxic effects (RR 2.10, 95% CI 1.30-3.38) and fatigue (RR 1.34, 95% CI 1.16-1.54)
Thus, treatment optimization is essential. In Europe, the EMA established the Cancer Medicines Forum with academia to optimize cancer treatments in clinical practice, and in Canada there is the Optimal Cancer Care Alliance. The goals of these entities is to maintain theoretical overall survival loss within acceptable boundaries, reduce toxicities, improve quality of life, and reduce cost. Interrupting ADT, the ARTA, or both is perceived as an attractive concept as part of a de-escalation strategy. There are several ways to potential go about de-escalation. The first includes stopping the androgen deprivation and continuing the ARTA:
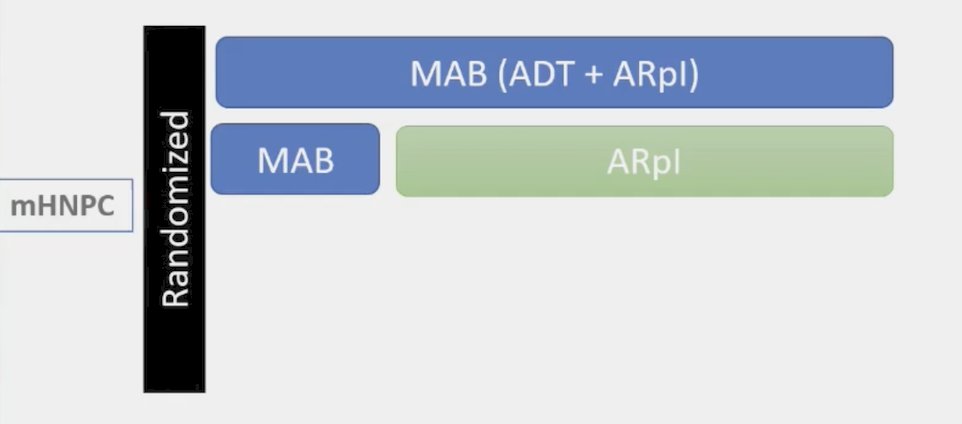
This is being assessed in the LIBERTAS study whereby newly diagnosed mHSPC patients are treated with apalutamide for 6 months following by an assessment based on PSA less than or greater than 0.2 ng/mL. For those with PSA >= 0.2 ng/mL, they continue on standard therapy, and those with PSA <0.2 ng/mL, they are randomized to apalutamide + intermittent ADT (Arm A) versus apalutamide + continuous ADT (Arm B). The trial design is as follows:
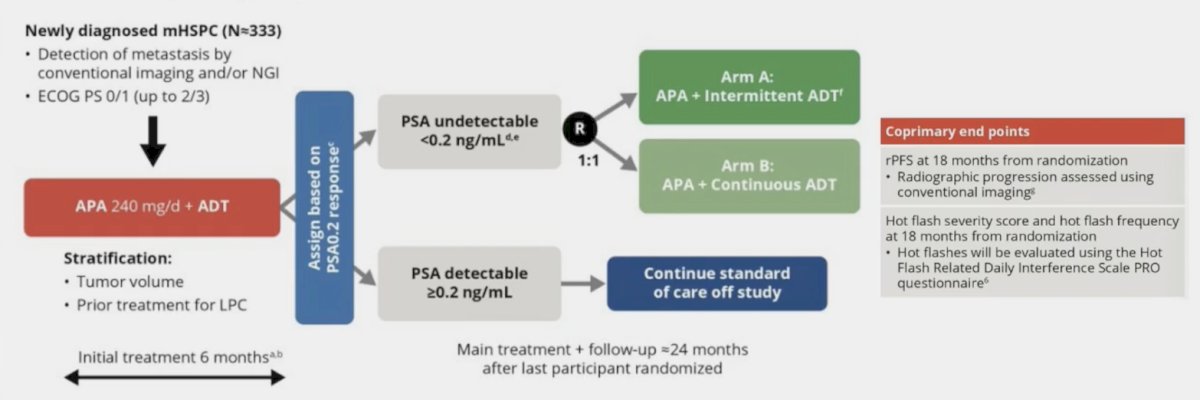
Based on EMBARK,1 we have seen improved outcomes with enzalutamide in high-risk biochemical recurrence, with this trial building in a treatment pause for appropriate responders. However, side effects are not numerically inferior with enzalutamide monotherapy:
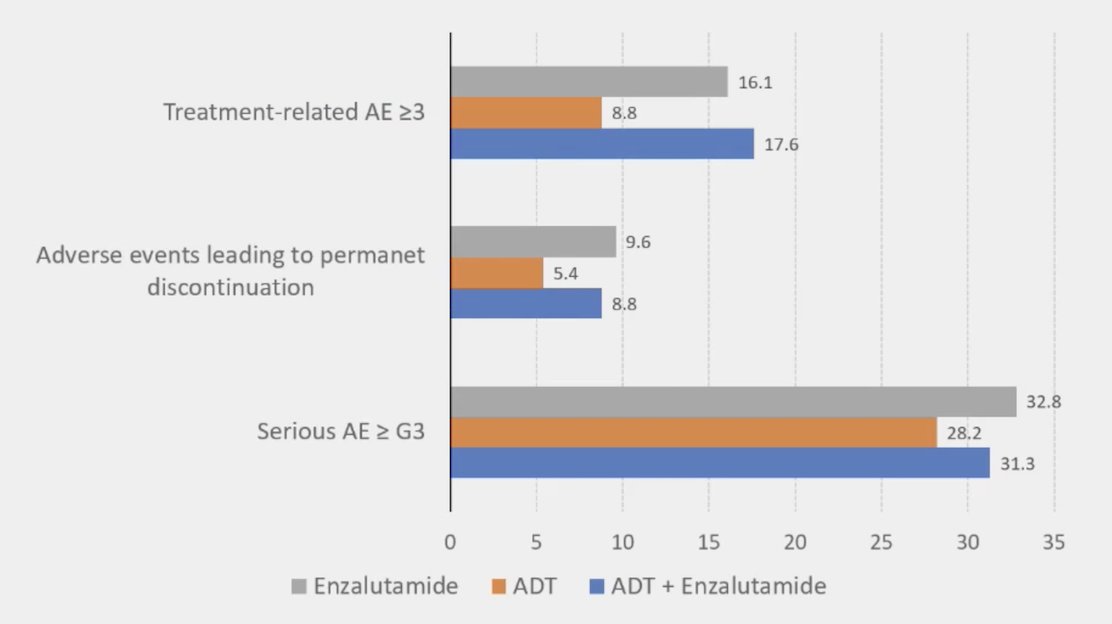
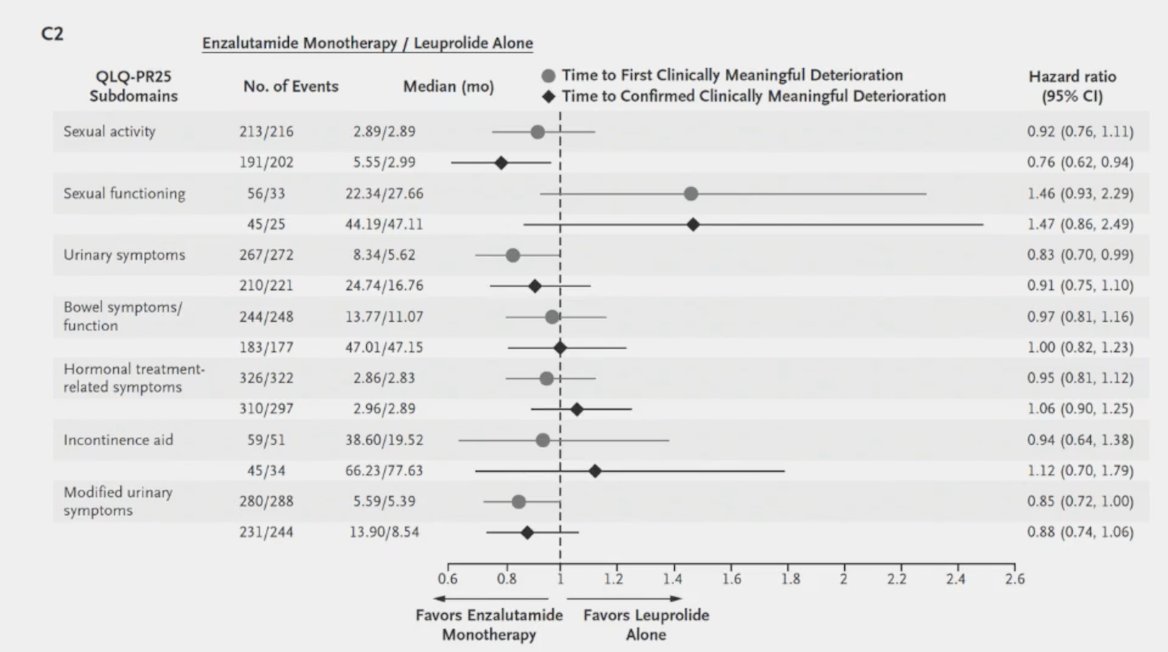
So, when considering a de-escalation trial where we maintain the ARTA but remove ADT, there is the potential for loss of overall survival, likely a negligible change in adverse events, potentially an improvement in quality of life (although uncertain), and no change in cost.
Dr. Tombal then discussed a de-escalation strategy whereby the ARTA is stopped and ADT is continued. This is being evaluated in the DUOS apalutamide/enzalutamide-short study in which men with low volume mHSPC are given ARTA treatment for 12 weeks followed by randomization to stopping the ARTA (n = 200) versus continuing the ARTA (n = 200):
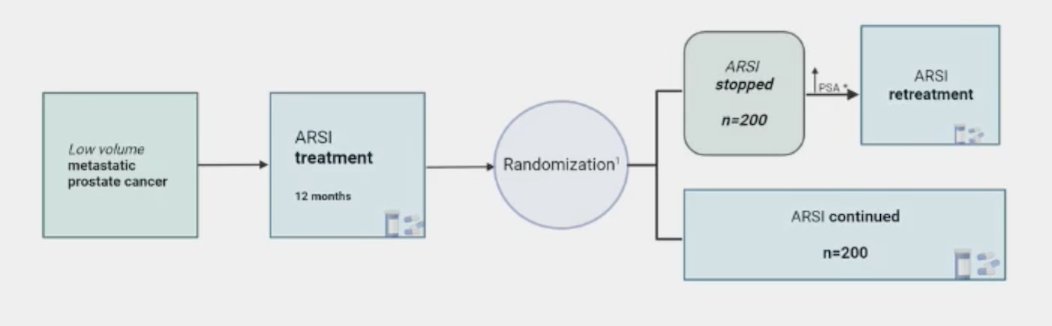
Key endpoints will be time to PSA progression and time to first-line treatment for mCRPC. Correlations between ctDNA levels and PSA will be undertaken, and quality of life will be assessed with FACT-P and EQ-5-D. For this trial design, there is a potential for loss of overall survival benefit, a potential for improvement in adverse events, questionable change in quality of life, and improvement in cost.
Finally, Dr. Tombal discussed a trial design of intermittent maximum androgen blockade, which is being assessed in the DE-ESCALATE EORTC 2238 trial:
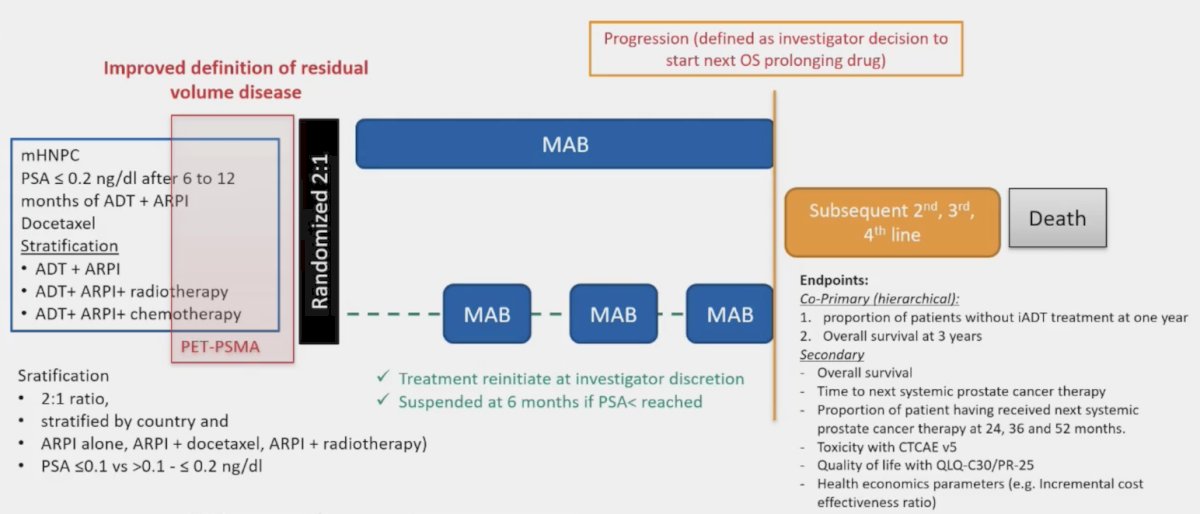
Intermittent ADT is an old approach, previously established by Hussain et al.2 in the SWOG-9346 trial. In this trial, 3,040 patients with mHSPC with a PSA of >= 5 ng/mL were treated with 7 months of goserelin + bicalutamide, and after 7 months of treatment, 1,535 patients achieved a PSA of < 4.0 ng/mL. The HR for death for intermittent androgen deprivation was 1.10 (90% CI 0.99-1.23) when compared to continuous androgen deprivation:
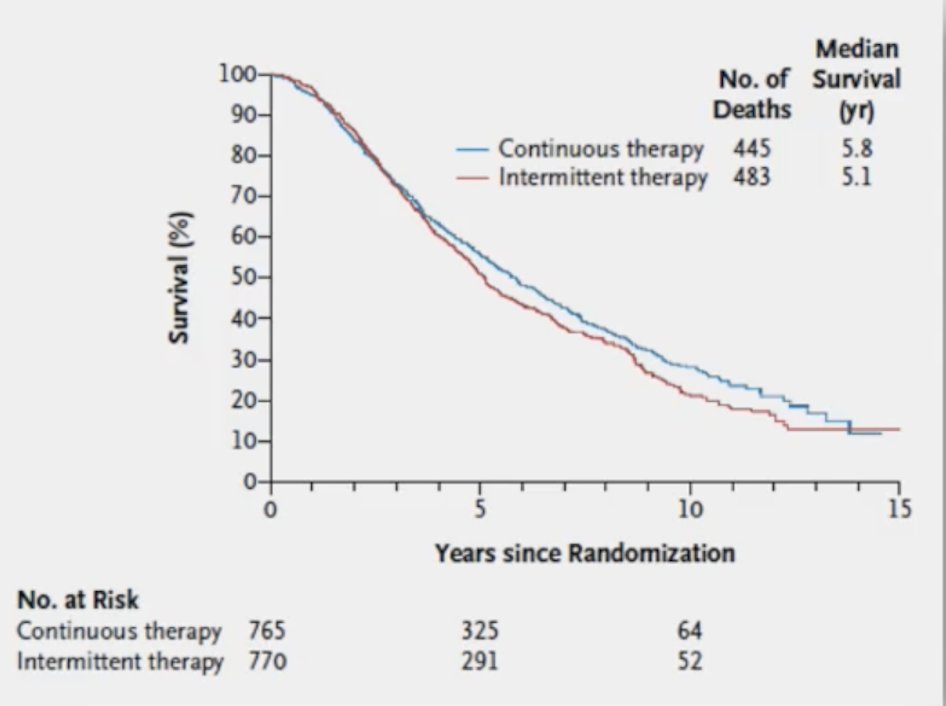
This approach was associated with improved quality of life, specifically with regards to sexual quality of life. Dr. Tombal emphasized that these types of de-escalation approaches should be tested in properly designed clinical trials. In Europe, he notes that, the Discrete Choice Experiment is being conducted to understand patient’s assessment of the benefits and risks of intermittent versus continuous ADT. Phase 1 includes qualitative interviews with patients to define the most critical quality of life attributes for survival and quality of life trade off. Additionally, this phase will seek feedback on how to express survival (ie. translating non-inferiority margins to the probability of survival). Phase 2 includes the discrete choice experiment survey to derive (or validate) non-inferiority thresholds based on the patient input to complement the EORTC 2238 DE-ESCALATE trial. The survey will be distributed by the patient organization Europa Uomo.
Dr. Tombal notes that the health related quality of life endpoint assessed by QOL-C30 and PR-25 are known to have limited value in patients receiving ADT. Additional items, such as EORTC IL-249 (physical, libido, pain, etc) have been extracted from the EORTC item bank and selected by patients for relevance.
Dr. Tombal concluded his presentation by discussing the patients with mHSPC that can be offered a treatment break and how to manage this break with the following take-home messages:
- It is not us, the physicians, but the patients, who must decide what overall survival loss is acceptable. What side effects are bothersome, and what domain of health related quality of life do they value most?
- New molecular imaging modalities should not be used at this point in time outside of clinical trials
- Offering treatment de-escalation to patients is an attractive concept. However, before doing so, we need definitive evidence rather than conventional wisdom
Presented by: Bertrand Tombal, MD, MPH, Institut de Recherche Clinique, Université Catholique de Louvain, Brussels, Belgium
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 Advanced Prostate Cancer Consensus Conference (APCCC) Meeting, Lugano, Switzerland, Thurs, Apr 25 - Sat, Apr 27, 2024.
References:
- Freedland SJ, de Almeida Luz M, De Giorgi U, et al. Improved Outcomes with Enzalutamide in Biochemically Recurrent Prostate Cancer. N Engl J Med 2023 Oct 19;389(16):1453-1465.
- Hussain M, Tangen CM, Berry DL, et al. Intermittent versus continuous androgen deprivation in prostate cancer. N Engl J Med 2013;368:1314-1325.


