(UroToday.com) The 2024 Advanced Prostate Cancer Consensus Conference (APCCC) meeting featured a session on the management of metastatic hormone sensitive prostate cancer (mHSPC), and a presentation by Dr. Oliver Sartor discussing the patients with mHSPC that should receive “total therapy”. In patients with low volume by conventional imaging per CHAARTED/STAMPEDE, Dr. Sartor notes that PSMA PET introduces huge heterogeneity in “low volume” patients:
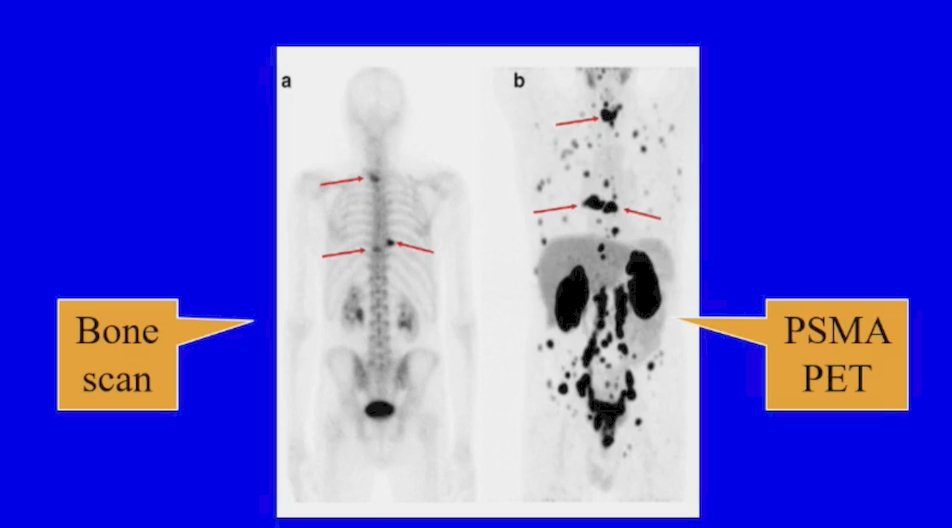
As such, Dr. Sartor notes that “is it not time to accept findings from the past and pivot toward the future?” Dr. Sartor highlighted that molecular imaging is the current and future reality. Can we agree on the definition of oligometastatic as 5 or fewer metastases? Or is this old school based on conventional imaging?
Total therapy may comprise:
- External beam radiation:
- Prostate +/- pelvic nodes
- Stereotactic body radiotherapy to the metastases
- ADT
- ARPIs: abiraterone, enzalutamide, apalutamide, or darolutamide
- Chemotherapy: docetaxel
However, Dr. Sartor asks: Does “total therapy” include all of the above, part of the above, or one of the above?
Dr. Sartor then provided a high-level summary of the data in the low volume metastatic subset:
- ADT + radiation to the prostate versus ADT – STAMPEDE
- PEACE-1 addresses the ADT + ARPI + radiation questions
- ADT + docetaxel versus ADT – CHAARTED/STAMPEDE/GETUG
- There is a mixed bag for “low volume” disease but some positives for “metachronous”
- ADT + ARPI versus ADT – there are numerous phase 3 trials
- ARPIs add value every time
- ADT + docetaxel + ARPI versus ADT + docetaxel
- ARASENS and PEACE-1 reported differently on overall survival
- ADT + ARPI + docetaxel versus ADT + ARPI
- No data (n = 0) to show that docetaxel adds value
- ADT + ARPI + metastasis directed therapy versus ADT + ARPI
- No phase III data
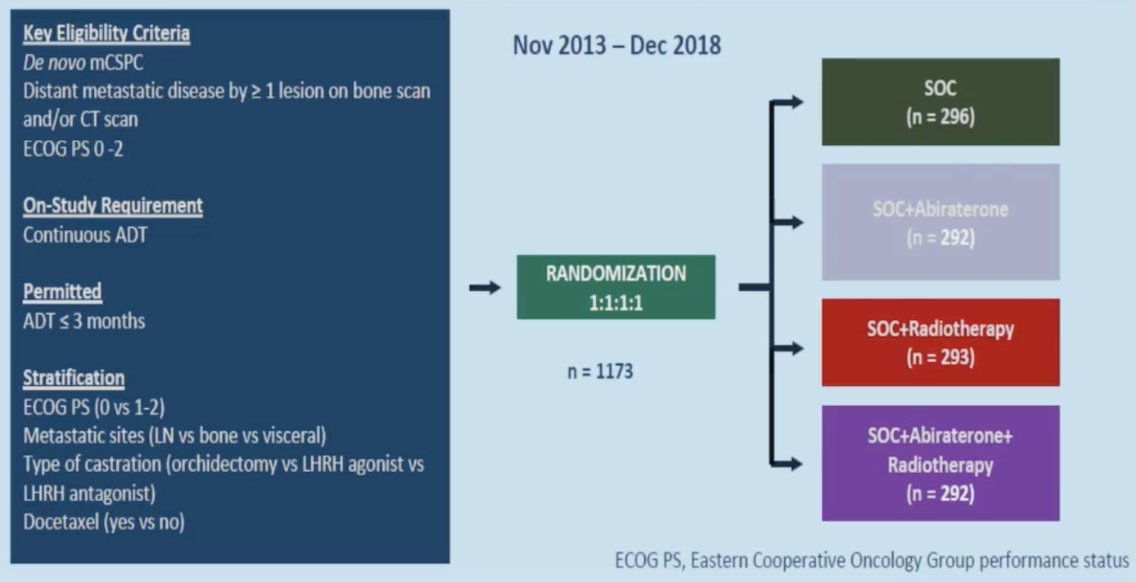
Dr. Sartor then discussed the PEACE-1 trial,1 a phase 3 trial with a 2x2 factorial design in men with de novo metastatic castration-sensitive prostate cancer: overall survival with abiraterone acetate + prednisone. The trial design for PEACE-1 is as follows:
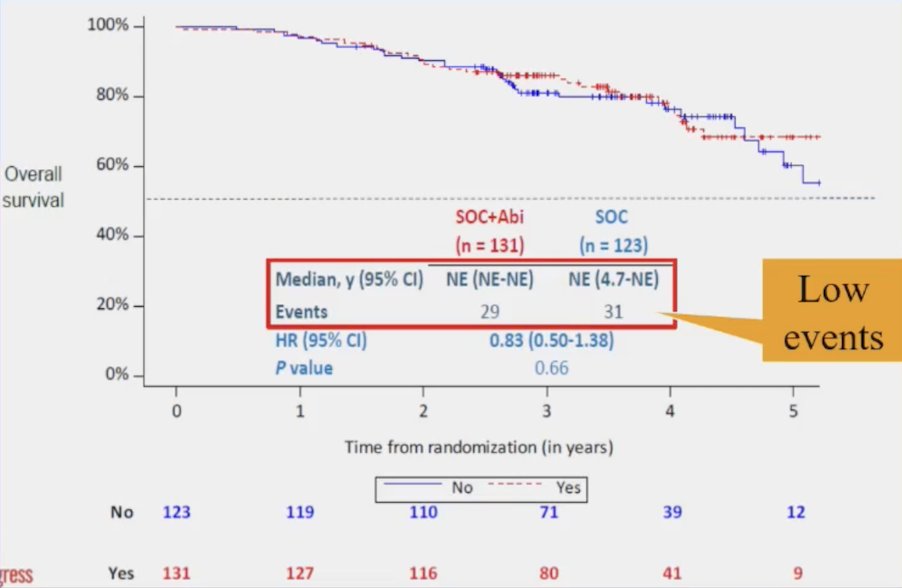
PEACE-1 overall survival in the “low volume” subset had very few events (n = 29 standard of care + abiraterone vs n = 31 standard of care) with no appreciable benefit for treatment intensification:
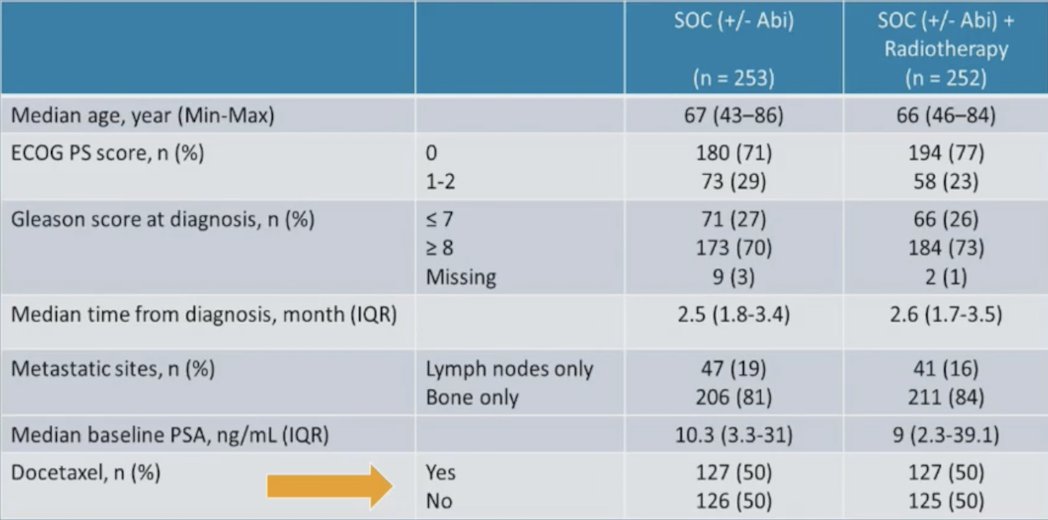
Additionally, there were no significant differences in baseline characteristics, stratified by receipt of radiotherapy (yes/no). Of note, 50% of patients in the low-volume cohort had received docetaxel:
Dr. Sartor notes that rPFS outcomes in the low volume population, stratified by the treatment arm showed that the addition of prostate radiotherapy to standard of care + abiraterone was associated with significant rPFS benefits (median 7.5 versus 4.4 years, p = 0.02):
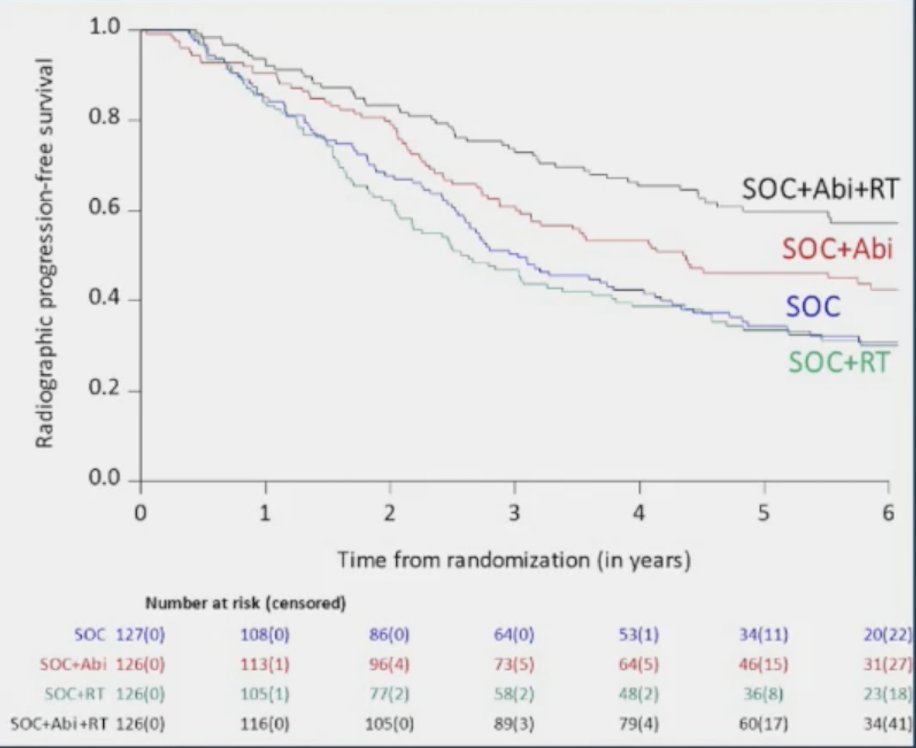
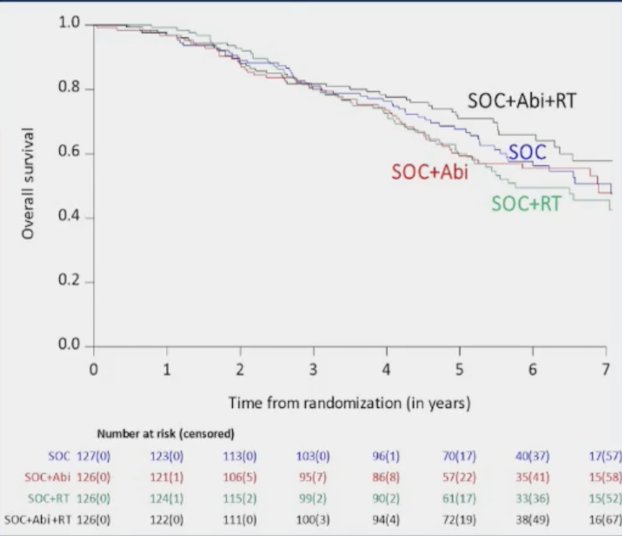
In the standard of care + abiraterone arms, the addition of prostate radiotherapy was associated with modest, non-significant overall survival benefits (HR 0.77, 95% CI 0.51 – 1.16, p = 0.21):
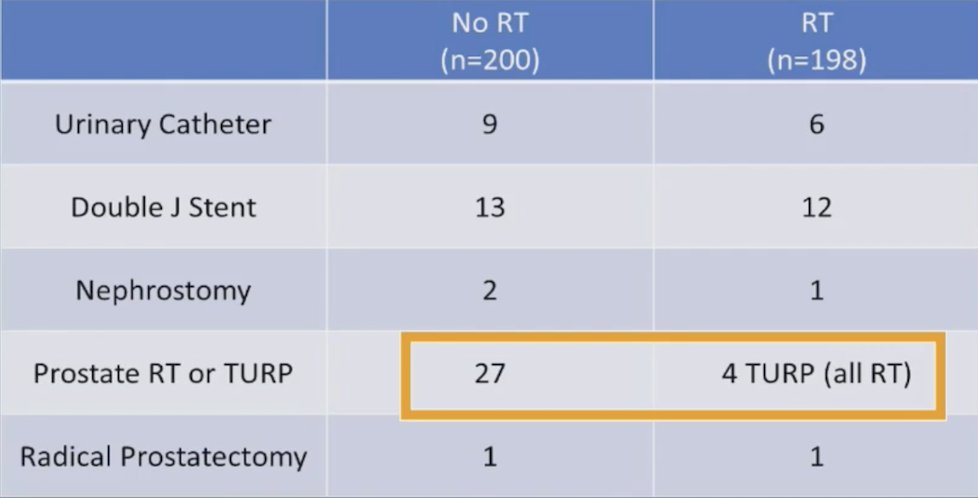
With regards to serious genitourinary events in the low volume population, those that received radiotherapy had lower incidence of prostate radiotherapy or TURP compared to those receiving no radiotherapy (4/198 vs 27/200). Thus, a potential benefit for radiotherapy in low volume patients includes a decrease in local symptoms:
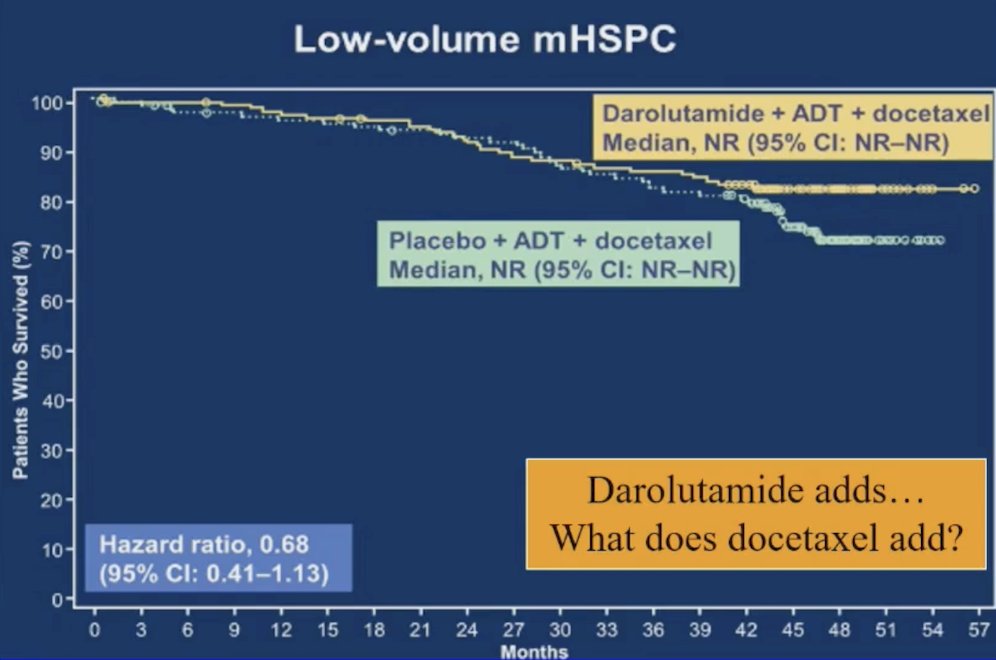
Dr. Sartor then discussed the ARASENS trial, which assessed darolutamide + docetaxel + ADT versus placebo + docetaxel + ADT, recently publishing overall survival by disease volume. ARASENS noted a potential clinical benefit, but no statistically significant benefit (HR 0.68, 95% CI 0.41-1.13) for overall survival in patients with low volume disease. Thus, Dr. Sartor emphasized that darolutamide adds benefit to triplet therapy, but what does docetaxel add?
Finally, Dr. Sartor discussed the EXTEND trial,3 which assessed the addition of metastasis directed therapy to intermittent hormone therapy for oligometastatic prostate cancer in a phase 2 setting. This trial showed that progression free survival was improved with combined therapy versus hormone therapy alone (HR 0.25, 95% CI 0.12-0.55):
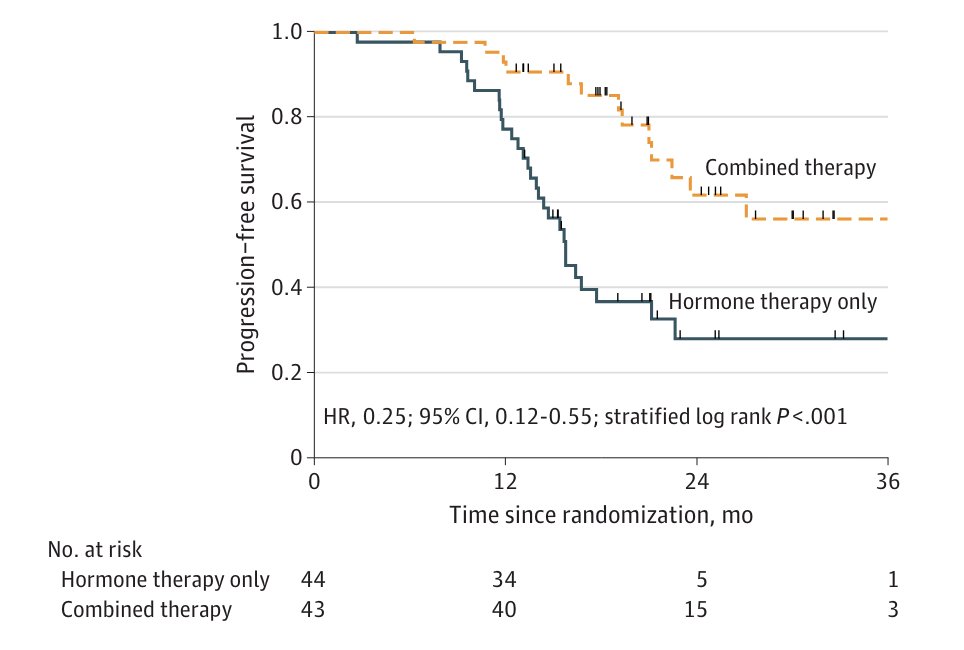
Dr. Sartor concluded his presentation by discussing synchronous low-volume patients with mHSPC that should receive “total therapy” with the following take-home messages:
- ARPIs + ADT adds value over ADT alone
- Docetaxel has no clear value added to ADT + ARPI
- Radiation to the prostate improves local control issues in ADT + ARPI patients based on the PEACE-1 data
- Metastasis directed therapy with stereotactic body radiotherapy seems like a good idea, but there are no phase 3 trials
- Radiation treatment to the prostate and all lesions in oligometastatic patients may be more important in the future when using no ADT or for intermittent therapy
Presented by: Oliver Sartor, MD, Mayo Clinic, Rochester, MN
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 Advanced Prostate Cancer Consensus Conference (APCCC) Meeting, Lugano, Switzerland, Thurs, Apr 25 - Sat, Apr 27, 2024.
References:
- Fizazi K, Foulon S, Carles J, Roubaud G, et al. Abiraterone plus prednisone added to androgen deprivation therapy and docetaxel in de novo metastatic castration-sensitive prostate cancer (PEACE-1): A multicentre, open-label, randomized, phase 3 study with a 2 x 2 factorial design. Lancet. 2022 Apr 30;399(10336):1695-1707.
- Hussain M, Tombal B, Saad F, et al. Darolutamide plus androgen-deprivation therapy and docetaxel in metastatic hormone-sensitive prostate cancer by disease volume and risk subgroups in the phase III ARASENS trial. J Clin Oncol. 2023 Jul 10;41(20):3595-3607.
- Tang C, Sherry AD, Haymaker C, et al. Addition of metastasis-directed therapy to intermittent hormone therapy for oligometastatic prostate cancer: The EXTEND phase 2 randomized clinical trial. JAMA Oncol. 2023 Jun 1;9(6):825-834.


