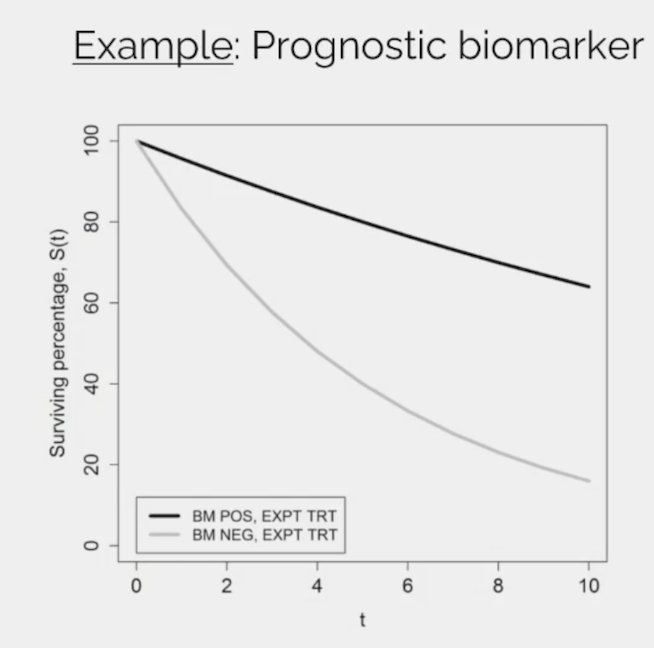
(UroToday.com) The 2024 Advanced Prostate Cancer Consensus Conference (APCCC) meeting featured a session on the management of metastatic hormone-sensitive prostate cancer (mHSPC), and a presentation by Dr. Matthew Smith discussing the relevant prognostic/predictive factors for the management of patients with mHSPC. Dr. Smith started by discussing prognostic biomarkers, which require a significant association between the biomarker and outcome:
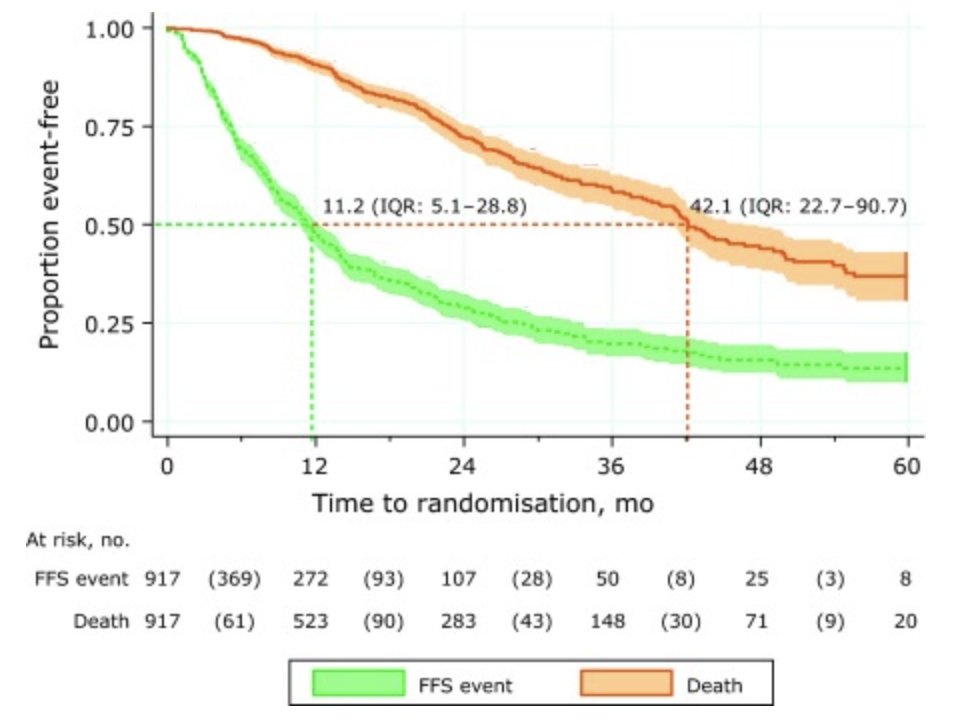
Dr. Smith then went on to discuss several influential examples of prognostic biomarkers in the mHSPC disease space. First, Dr. Smith highlighted the STAMPEDE platform and the experience with ADT.1 The goal of this analysis was to describe survival outcomes, along with current treatment standards and factors associated with prognosis. Among 917 men with M1 disease in the control arm, over a median follow-up of 20 months, most men (n = 574; 62%) had bone-only metastases, whereas 237 (26%) had both bone and soft tissue metastases, mainly in distant lymph nodes. Overall, there were 238 deaths, including 202 (85%) from prostate cancer. The median failure free survival was 11 months, with a 2-year failure free survival of 29% (95% CI, 25-33). The median overall survival was 42 months, with a 2-year overall survival rate of 72% (95% CI, 68-76):
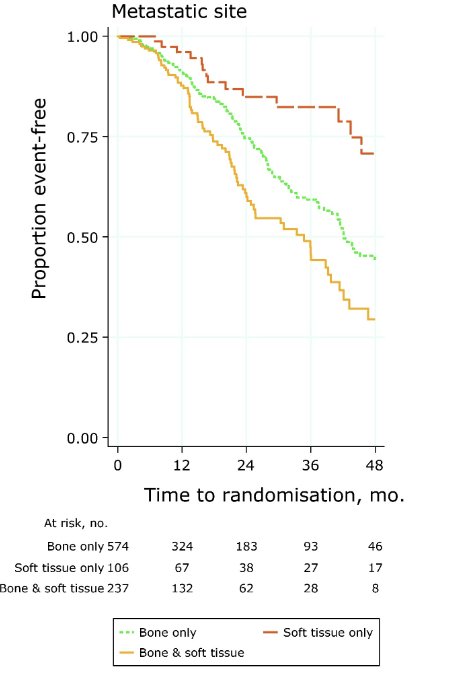
Overall survival stratified by bone only metastasis, soft tissue only metastasis, and bone + soft tissue metastasis is as follows:
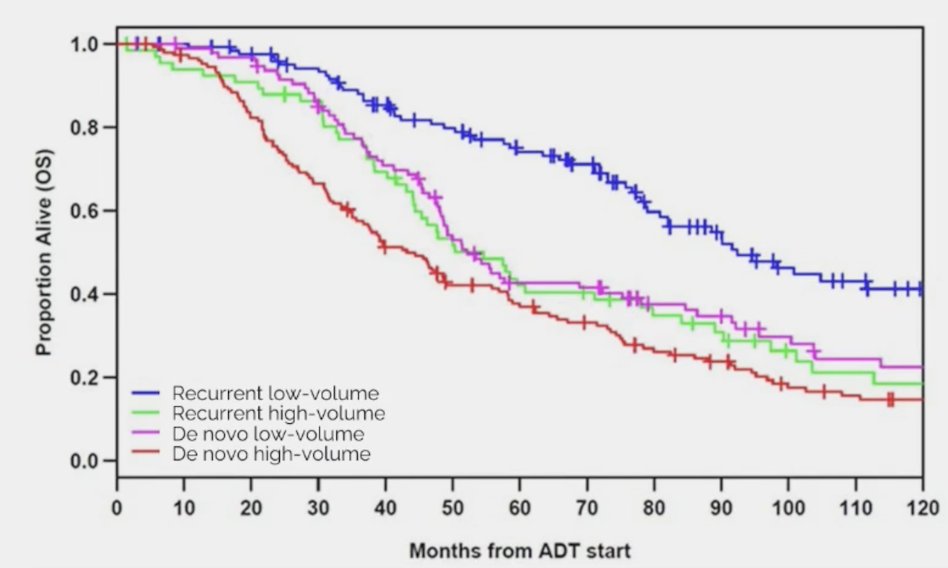
Next, Dr. Smith highlighted overall survival and disease volume at presentation. Francini et al. 2 assessed whether a classification based on time of metastatic disease (after prior local therapy vs de novo) and disease volume (low volume vs high volume) are prognostic for mHSPC patients treated with ADT. Among 436 patients treated at the Dana Farber Cancer Institute included in the analysis, the median overall survival for patients after prior local therapy/low volume disease was 92.4 (95%CI: 80.4-127.2) months and 43.2 (95%CI: 37.2-56.4) months for de novo/high volume disease, whereas intermediate values were observed for after prior local therapy/high volume disease and de novo/low volume disease. A robust gradient for both outcomes was observed (Trend test p < 0.0001) in the four groups:
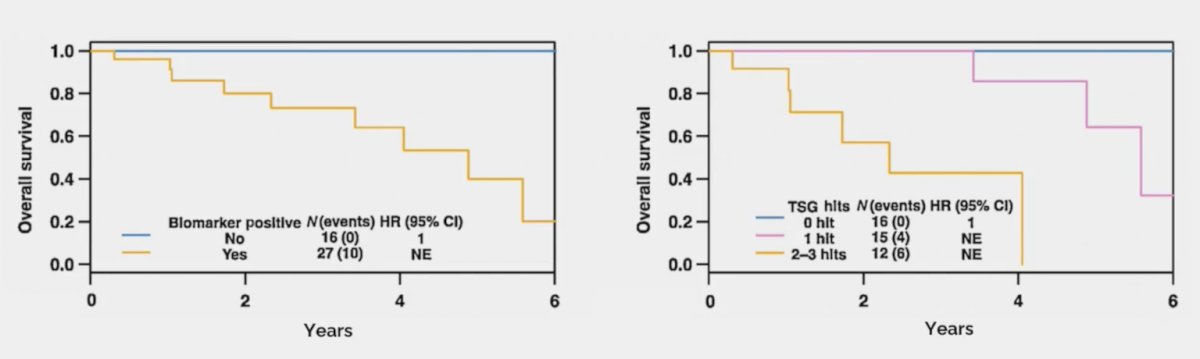
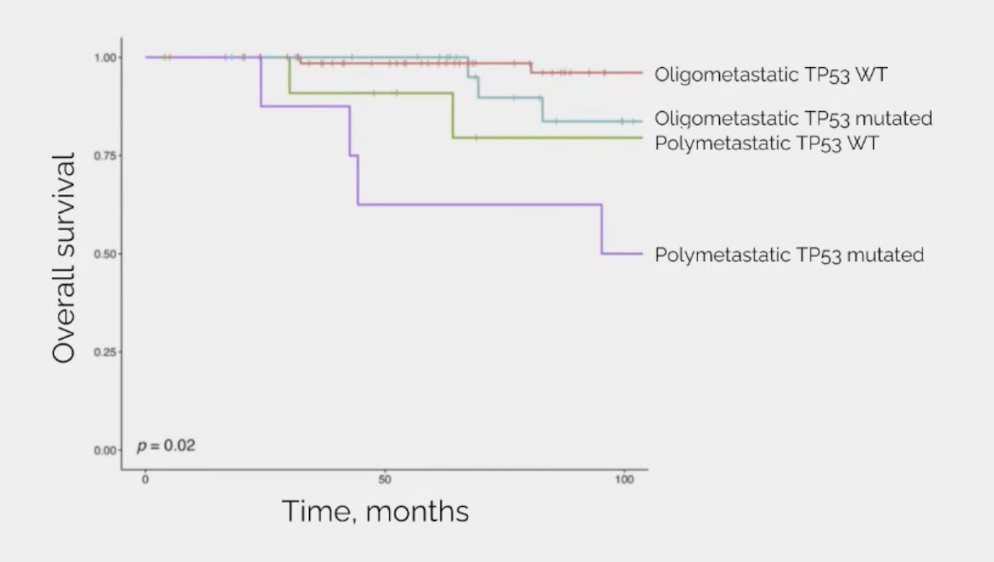
Additionally, there is an emerging biological understanding of the disease with increasing mutation frequency with increasing “aggressiveness”. In a retrospective study of men with mHSPC who underwent sequencing of their tumors, the frequency of driver mutations in TP53 (p = 0.01), WNT (p = 0.08), and cell cycle (p = 0.04) genes increased across the mHSPC spectrum.2 TP53 mutational status and overall survival is as follows:
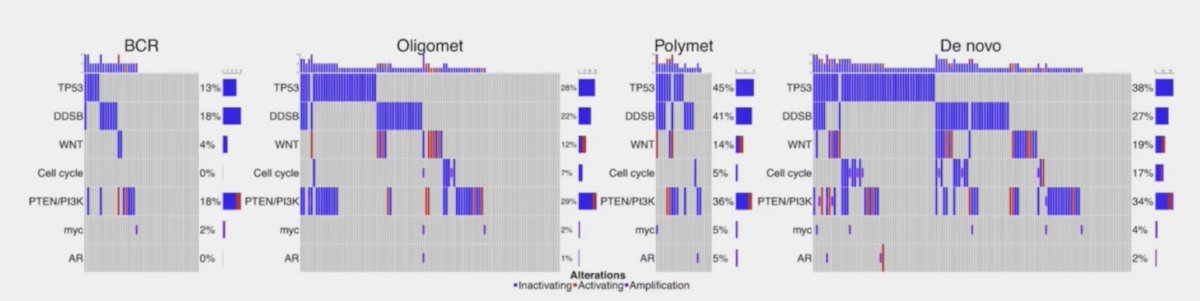
Dr. Smith summarized the emerging biological understanding and treatment impact as follows:
Compound genomic alterations of TP53, PTEN, and RB1 tumor suppressors are also prognostic of overall survival in mHSPC:3
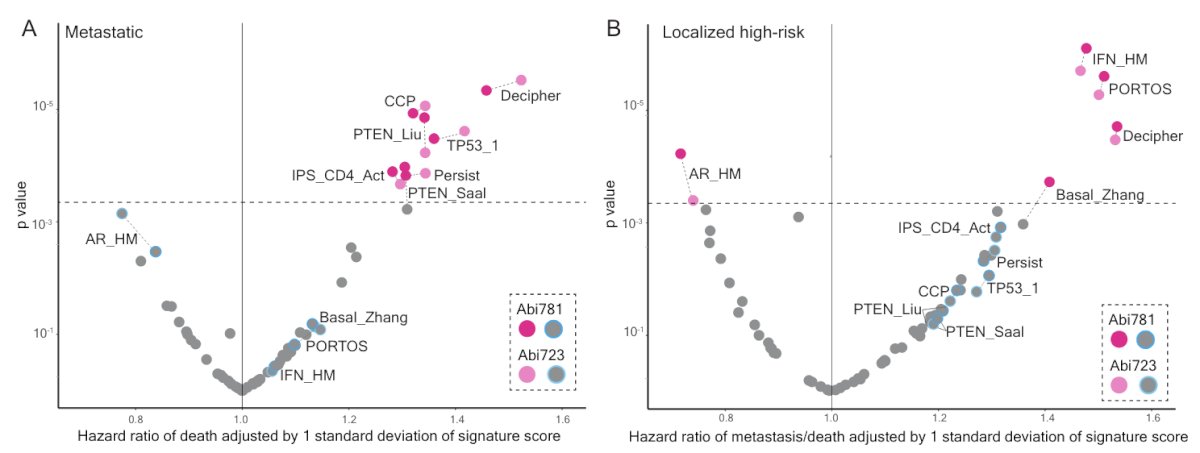
Dr. Smith then discussed work from Dr. Attard’s group regarding an ancillary study of the STAMPEDE abiraterone phase 3 trial assessing clinical testing of transcriptome-wide expression profiles in high-risk localized and metastatic prostate cancer starting ADT.4 The following represents a scatter plot of hazard ratios (adjusted by one standard deviation of signature score) and p values resulting from prognostic testing adjusted for clinical and pathological variables of overall survival in 57 signatures (with continuous scores) in patients with metastatic and localized disease:
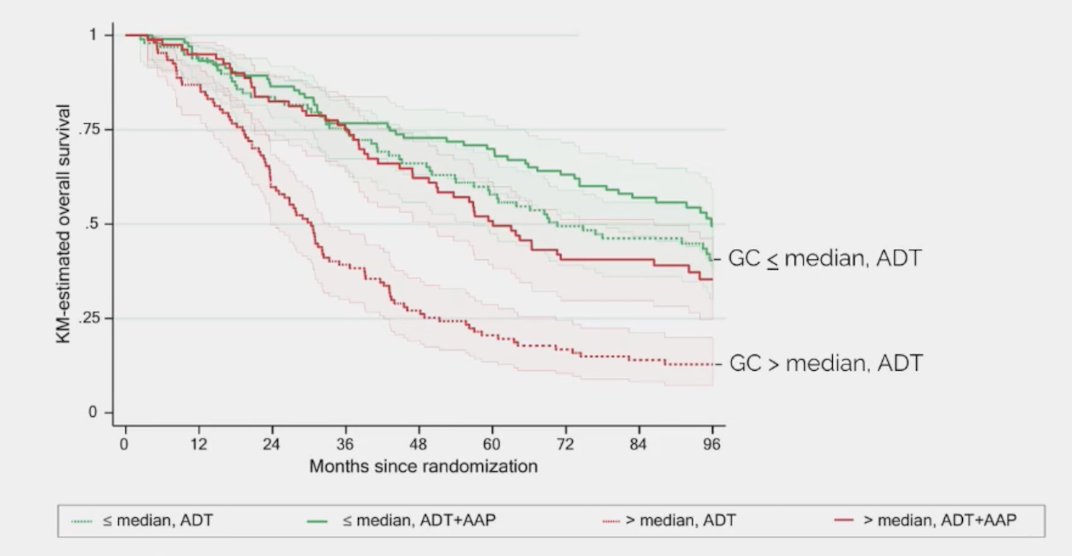
This same study also assessed the Decipher genomic classifier and overall survival in mHSPC. The following figure highlights a Kaplan-Meier plot of overall survival in patients with metastatic disease in Abi781 receiving either ADT or ADT + abiraterone + prednisone split by Decipher score above or below median (0.77 on scale of 0- 1):
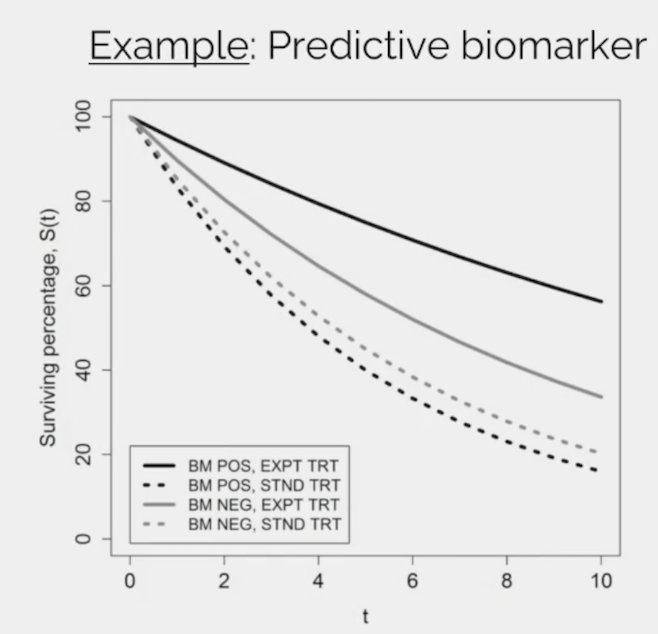
Dr. Smith then discussed predictive biomarkers, which require data from a randomized controlled trial and a significant biomarker-treatment effect interaction:
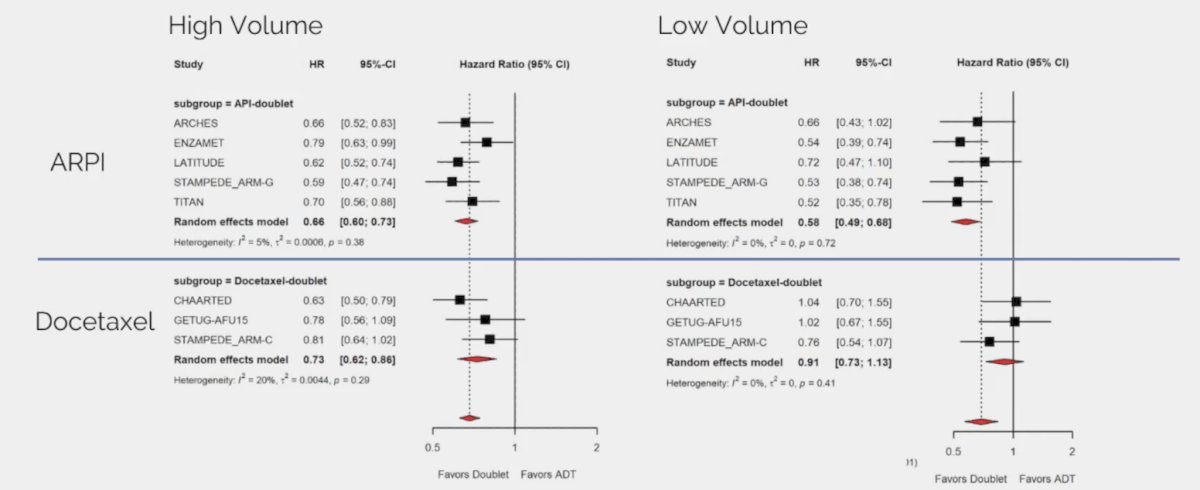
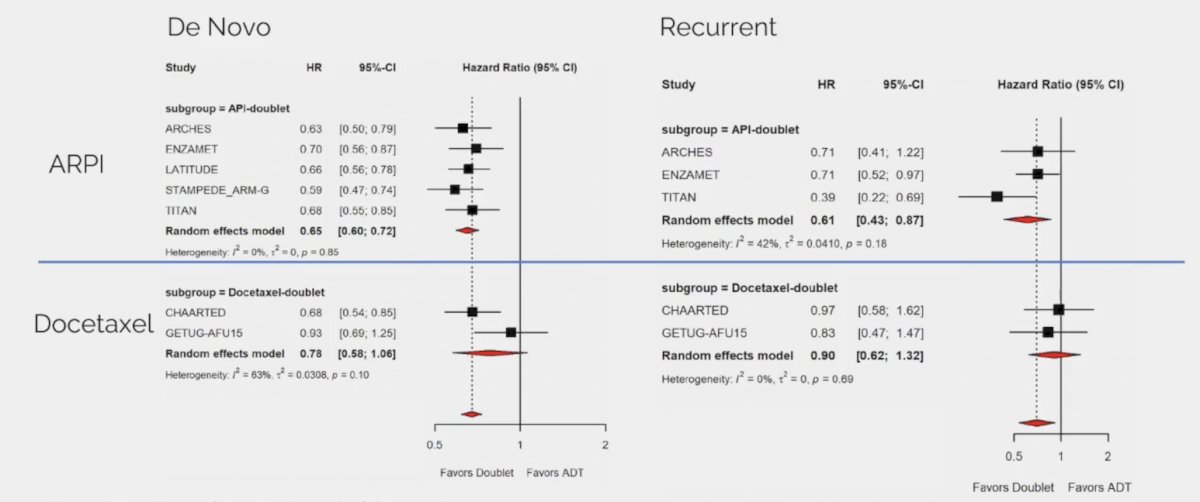
Although there are no true predictive biomarkers in mHSPC, Dr. Smith notes that there are several relevant scenarios that provide thought provoking data. The first is overall survival in mHSPC by disease volume and disease presentation for ARPI doublet and docetaxel doublet therapy:
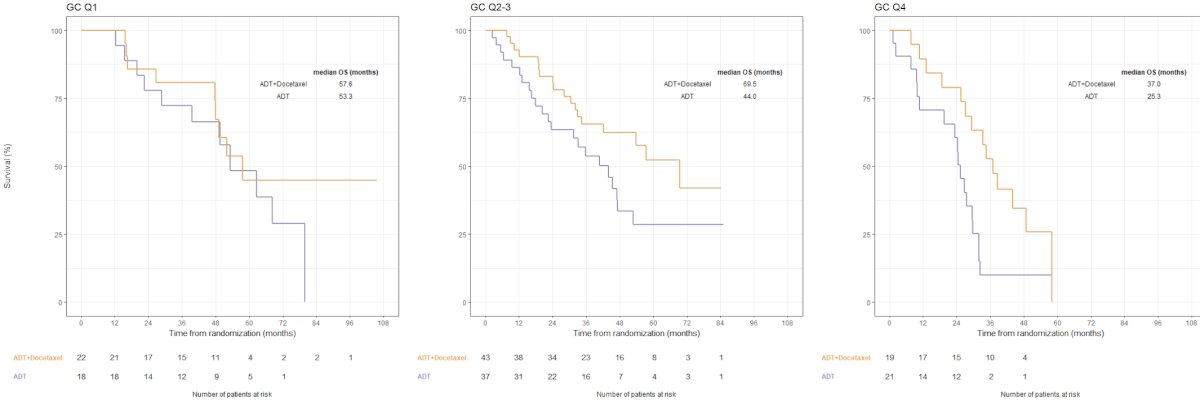
Hamid and colleagues5 previously looked at the effect of docetaxel on overall survival utilizing the Decipher score in the CHAARTED trial. The effect of docetaxel on overall survival was observed across all genomic classifier groups; however, the relative benefit of chemohormonal therapy varied by genomic classifier group was significant with higher genomic classifier (higher risk) disease (Q1: HR 0.72, 95% CI 0.29-1.73; Q2-3: HR 0.57, 95% CI 0.30-1.05; and Q4: HR 0.41, 95% CI 0.19-0.84). This can be represented as an absolute benefit in overall survival for addition of docetaxel to ADT for men with tumors in genomic classifier Q1 versus genomic classifier Q4 of 9% versus 25% at 3 years, respectively:
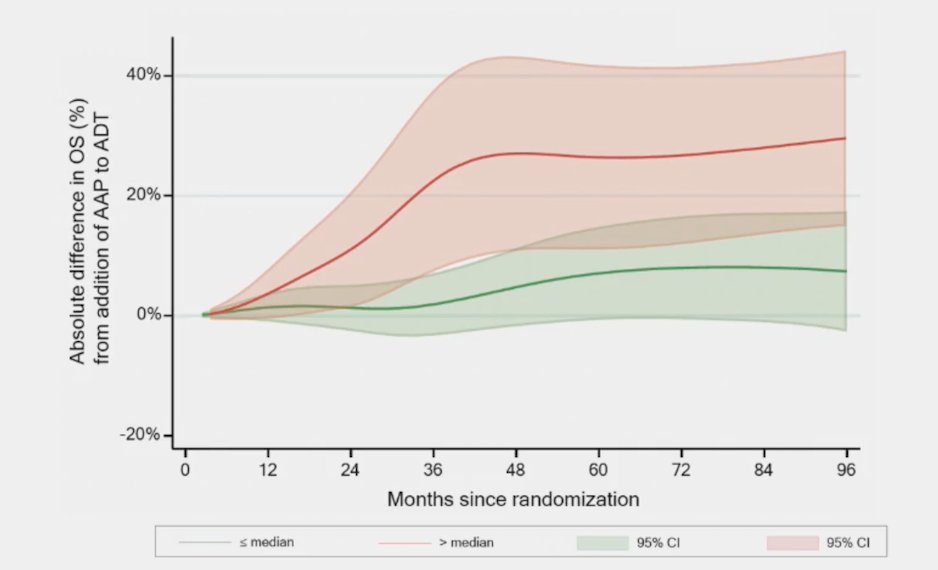
Finally, Dr. Smith noted the line plot showing the absolute difference in overall survival (as a percentage) of the addition of abiraterone + prednisone to ADT in patients with metastatic disease in Abi781* split by Decipher score above or below median with 95% CIs, adjusted for clinical and pathological variables:
Dr. Smith concluded his presentation discussing the relevant prognostic/predictive factors for the management of patients with mHSPC with the following take-home messages:
- Disease presentation, anatomic pattern of metastases, and disease volume are associated with overall survival in mHSPC
- Certain single gene alterations, including PTEN and TP53, are associated with shorter overall survival
- High Decipher scores are associated with shorter overall survival
- There are no predictive biomarkers in mHSPC
Presented by: Matthew R. Smith, MD, PhD, Massachusetts General Hospital Cancer Center, Boston, MA
Written by: Zachary Klaassen, MD, MSc - Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 Advanced Prostate Cancer Consensus Conference (APCCC) Meeting, Lugano, Switzerland, Thurs, Apr 25 - Sat, Apr 27, 2024.
References:
- James ND, Spears MR, Clarke NW, et al. Survival with Newly Diagnosed Metastatic Prostate Cancer in the “Docetaxel Era”: Data from 917 Patients in the Control Arm of the STAMPEDE TRIAL (MRC PR08, CRUK/06/019). Eur Urol. 2015 Jun;67(6):1028-1038.
- Francini E, Gray KP, Xie W, et al. Time of metastatic disease presentation and volume of disease are prognostic for metastatic hormone sensitive prostate cancer (mHPSC). Prostate. 2018 Sep;78(12):889-895.
- Hamid AA, Gray KP, Shaw G, et al. Compound Genomic Alterations of TP53, PTEN, and RB1 Tumor Suppressors in Localized and Metastatic Prostate Cancer. Eur Urol. 2019 Jul;76(1);89-97.
- Parry MA, Grist E, Mendes L, et al. Clinical testing of transcriptome-wide expression profiles in high-risk localized and metastatic prostate cancer starting androgen deprivation therapy: An ancillary study of the STAMPEDE abiraterone phase 3 trial. Res Sq. 2023 Feb 8 [Epub ahead of print].
- Hamid AA, Huang H-C, Wang V, et al. Transcriptional profiling of primary prostate tumor in metastatic hormone-sensitive prostate cancer and association with clinical outcomes: Correlative analysis of the E3805 CHAARTED trial. Ann Oncol. 2021 Sep;32(9):1157-1166.