(UroToday.com) The 2024 Advanced Prostate Cancer Consensus Conference (APCCC) meeting featured a session on the treatment for biochemical recurrence/PSA persistence, and a presentation by Dr. Neal Shore discussing whether some patients with biochemical recurrence, after either radiotherapy or radical prostatectomy, are candidates for androgen receptor antagonist monotherapy and how to manage the systemic therapy. Dr. Shore notes that his topic of discussion touches on several of the key questions proposed by the APCCC:
- For the majority of patients who receive enzalutamide or bicalutamide monotherapy (150mg daily), do you recommend a primary prophylaxis for gynecomastia?
- For the majority who receive enzalutamide or bicalutamide and who develop relevant gynecomastia, what further investigations do you recommend?
- For the majority who receive enzalutamide or bicalutamide and who develop bothersome gynecomastia, what is your preferred treatment?
- For the majority of patients who develop bothersome mastodynia, what is your preferred treatment?
- Is it appropriate to extrapolate the data generated with bicalutamide 150 mg on prophylaxis of gynecomastia to the AR antagonists (apalutamide, darolutamide, enzalutamide)?
Dr. Shore emphasized that in the EMBARK trial,1 the definition of non-metastatic hormone-sensitive prostate cancer with high-risk features was defined as follows:
- M0 by conventional imaging
- PSA doubling time less than or equal to 9 months
- PSA greater than or equal to 2 ng/ml above nadir after radiotherapy or
- PSA greater than or equal to 1 ng/ml after radical prostatectomy with or without postoperative radiotherapy, and
- Not considered a candidate for pelvic-directed therapy
The EMBARK trial was initially presented at AUA 2023, with the objective of evaluating enzalutamide in combination with leuprolide acetate and enzalutamide monotherapy in patients with high-risk biochemical recurrence. All patients had a PSA ≥1 ng/ml after radical prostatectomy or ≥2 ng/ml above nadir after primary external beam radiotherapy, with a PSA doubling time of ≤9 months. Patients had no evidence of metastasis on conventional imaging and baseline testosterone was ≥150 ng/dL. Hormone therapy ≥9 months prior to enrolment was permitted. Patients underwent stratified randomization (by PSA level, PSA doubling time, and prior hormonal therapy receipt) to one of three arms:
- Enzalutamide 160 mg (standard dose) + leuprolide acetate (blinded arm)
- Placebo + leuprolide acetate (blinded)
- Enzalutamide monotherapy (unblinded)
PSA was assessed at 36 weeks, and if patients had:
- PSA <0.2 ng/mL: Treatment was suspended at week 37 and PSA was monitored with treatment reinitiated if PSA rose again
- PSA>0.2 ng/mL: Treatment was continued
The study design is summarized below:
The primary endpoint was metastasis-free survival, assessed via blinded independent central review, in the enzalutamide + leuprolide versus leuprolide arms only. Key secondary endpoints included overall survival and safety outcomes. At a median follow-up of 5 years, the combination of enzalutamide/leuprolide, versus leuprolide alone, demonstrated a significant improvement in metastasis-free survival (HR 0.42, 95% CI 0.31 – 0.61, p<0.0001). The median metastasis-free survival was not reached in either arm as of date:
Next, comparisons between the enzalutamide monotherapy and leuprolide acetate monotherapy arms were performed. This demonstrated prolonged metastasis-free survival in the enzalutamide-only arm, with a HR of 0.63 (95% CI 0.46 – 0.87, p=0.0049):
Time to PSA progression and time to first use of new antineoplastic therapy were also prolonged in the enzalutamide versus leuprolide-only comparisons:
Dr. Shore then discussed gynecomastia, which is the benign proliferation of glandular tissue in the male breast beneath the nipple (subareolar region). True gynecomastia is typically a rubbery or firm mound of tissue that is concentric with the nipple-areolar complex. Gynecomastia is likely caused by an imbalance between increased estrogen activity and decreased androgen activity at the breast tissue level, and can be associated with many other conditions, such as: (i) primary or secondary gonadal failure, (ii) androgen resistance syndromes, (iii) hyperthyroidism, (iv) chronic liver disease, (v) use of some medications such as spironolactone, digoxin, bicalutamide, cimetidine, and (vi) drugs of abuse such as alcohol and marijuana.
Three trials have assessed enzalutamide monotherapy, with a combined 536 patients as summarized below: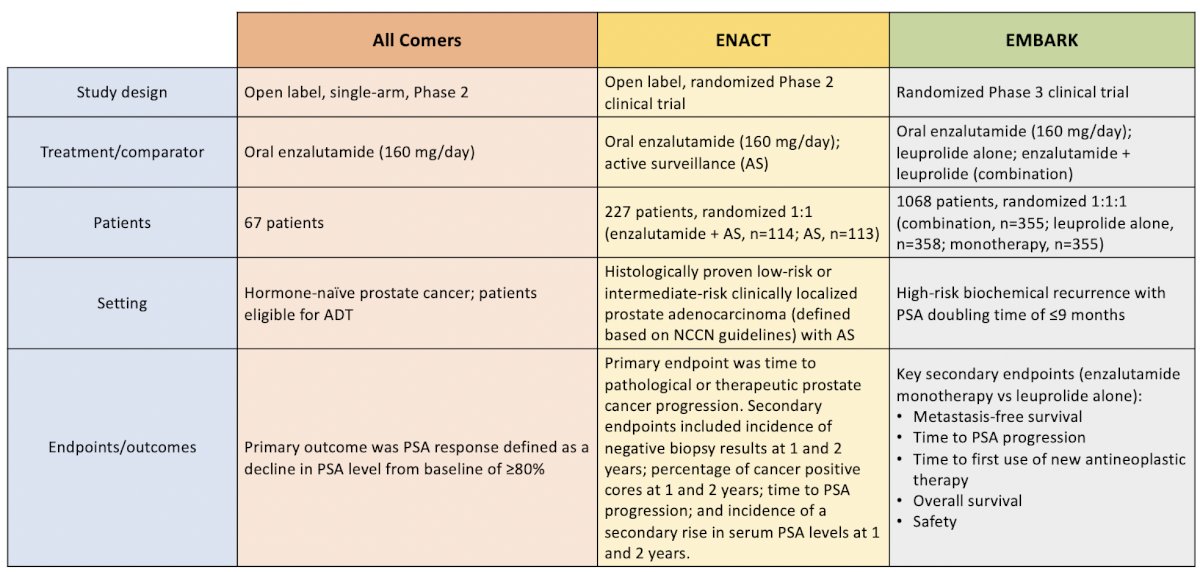
Importantly, in all three trials, there was no prophylactic treatment of adverse events for these patients. Moreover, treatment-emergent adverse events, including gynecomastia and nipple tenderness and pain, were not infrequent: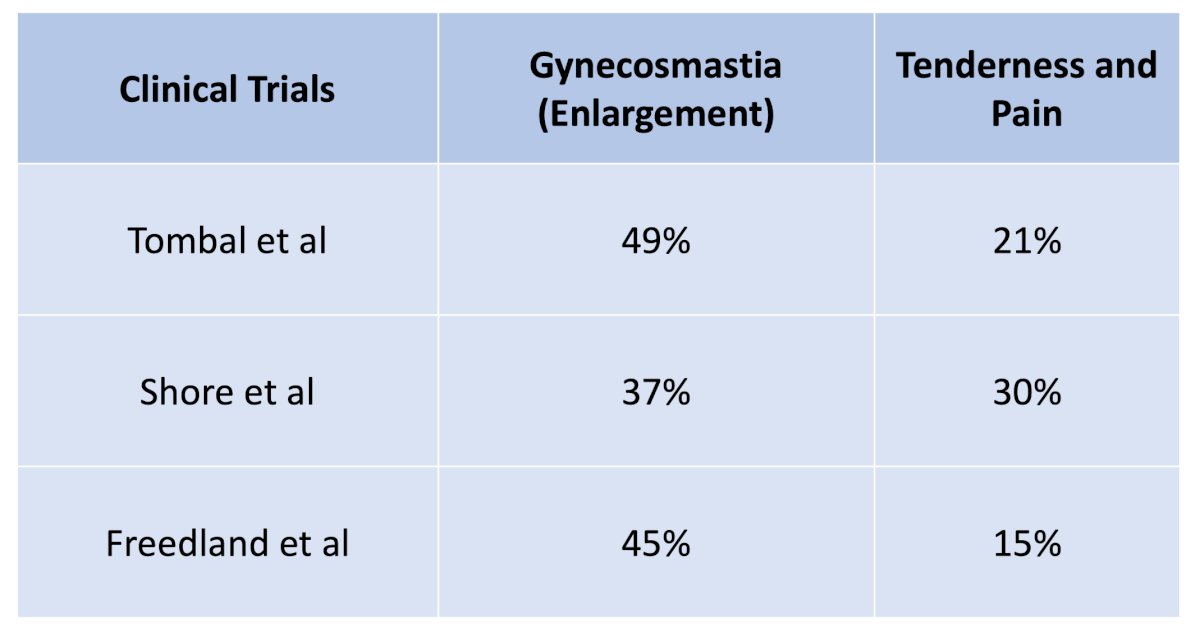
Two trials have evaluated the use of prophylactic radiotherapy for anti-androgen-related gynecomastia and breast pain: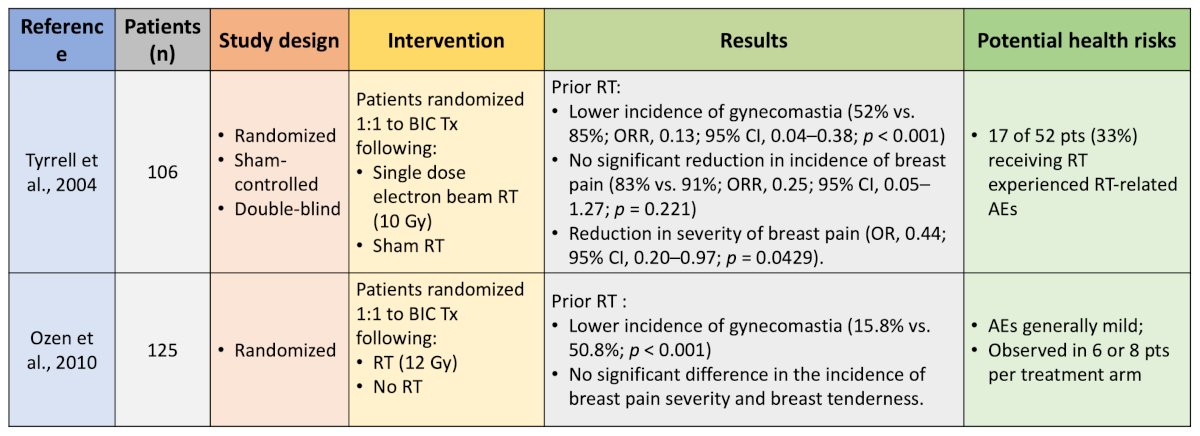
Dr. Shore notes that his takeaway from these trials are that a single dose of radiotherapy improves gynecomastia, was generally well tolerated, but did not significantly decrease breast pain or tenderness. Five studies have evaluated the prophylactic use of endocrine therapy for anti-androgen-related gynecomastia and breast pain, including the use of tamoxifen and anastrozole: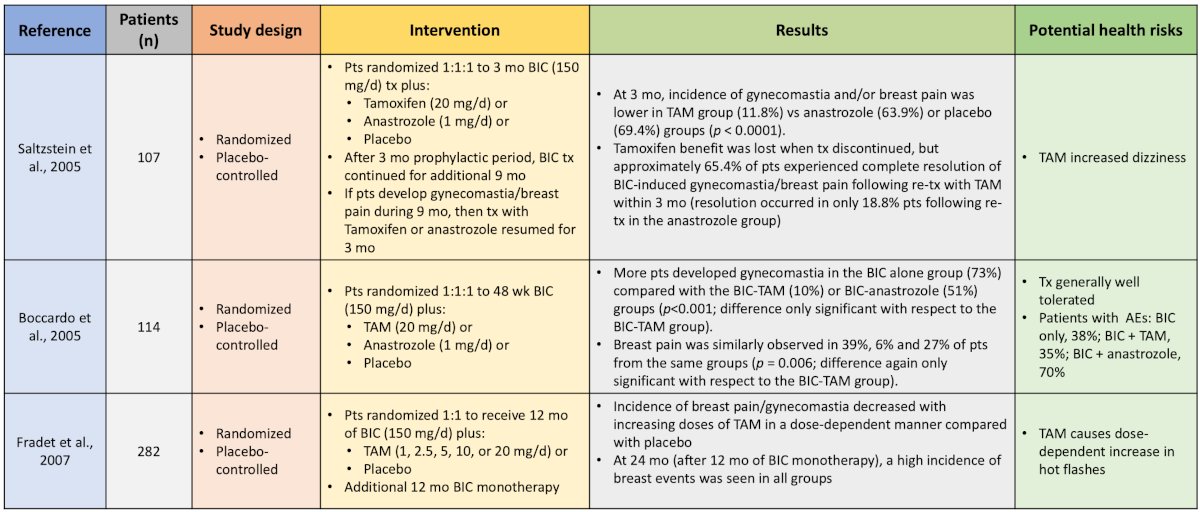
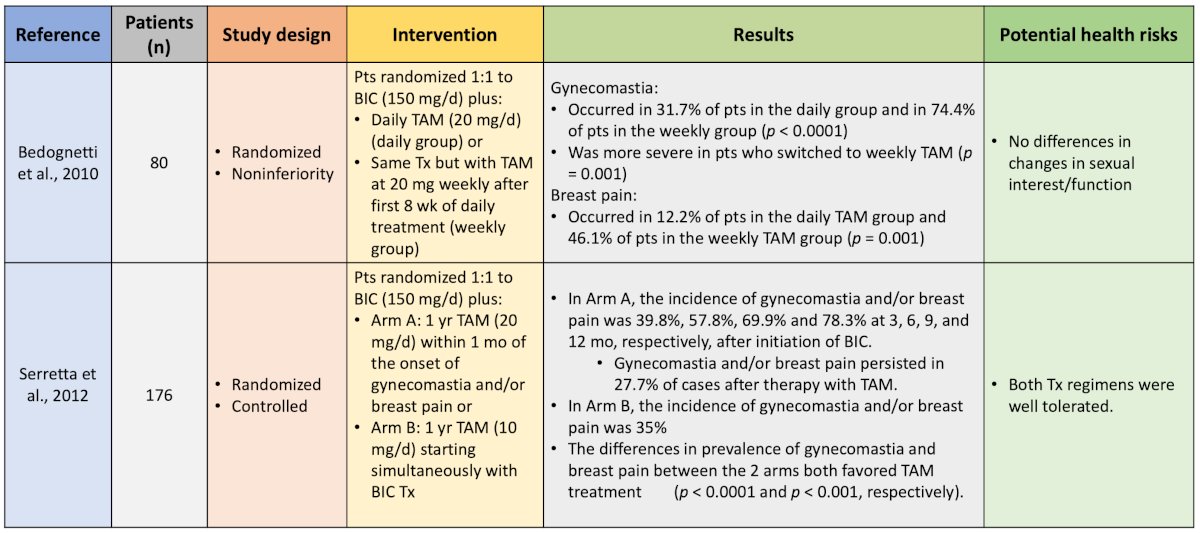
Generally, these studies showed that tamoxifen was better than anastrozole and better than placebo. Finally, two studies have evaluated prophylactic radiotherapy + tamoxifen for antiandrogen-related gynecomastia and breast pain: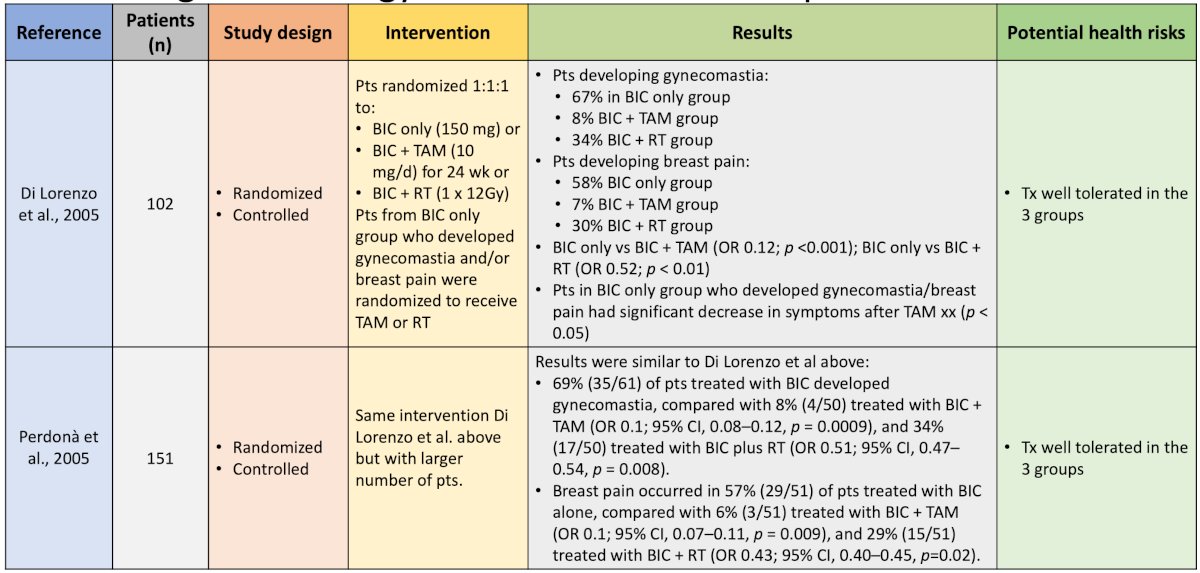
These trials support the combination of radiotherapy + tamoxifen as the most effective strategy for reducing all breast-related symptoms. A systematic review from Johnson and colleagues suggests that serum estrogen receptor modulators (ie. tamoxifen), aromatase inhibitors (ie. anastrozole), and radiotherapy or both prevent or reduce gynecomastia and breast pain associated with antiandrogen use. Of the three options, tamoxifen is the most effective.2 Dr. Shore's message is that tamoxifen is the least costly and most accessible, although we need to consider drug-drug interactions, compliance, and side effect issues.
Additional considerations include a surgical approach, which is the gold standard for symptomatic gynecomastia in most patients. Surgery can either be somewhat invasive, such as a subcutaneous mastectomy that involves the direct resection of the glandular tissue using a peri-areolar or trans-areolar approach with or without liposuction, or a less invasive technique utilizing an axillary approach and endoscopic visualization and resection. However, there is no high-quality evidence that exists to support one surgical approach over the other.
Dr. Shore concluded his presentation discussing whether some patients with biochemical recurrence, after either radiotherapy or radical prostatectomy, are candidates for androgen receptor antagonist monotherapy and how to manage the systemic therapy by answering the aforementioned APCCC questions related to this topic:
- For the majority of patients who receive enzalutamide monotherapy, do you recommend a primary prophylaxis for gynecomastia?
- Yes, knowing the options and with shared decision-making: radiotherapy and/or tamoxifen
- For the majority who receive enzalutamide who develop relevant gynecomastia, what further investigations do you recommend?
- Physical examination is easy/cost-effective (if doubtful, consider a mammogram)
- For the majority who receive enzalutamide who develop bothersome gynecomastia, what is your preferred treatment?
- If it is pain/tenderness: tamoxifen
- If it is enlargement: simple mastectomy
- For the majority of patients who develop bothersome mastodynia, what is your preferred treatment?
- Tamoxifen
- Is it appropriate to extrapolate the data generated with bicalutamide 150mg on prophylaxis of gynecomastia to the AR antagonists (apalutamide, darolutamide, enzalutamide)?
- Yes, as the mechanisms of action are relatively comparable, albeit prospective trials are always preferable
Presented by: Neal Shore, MD, FACS, Director, CPI, Carolina Urologic Research Center, Chief Medical Officer of Surgery and Oncology, GenesisCare USA, Atlantic Urology Clinics, Myrtle Beach, SC
Written by: Zachary Klaassen, MD, MSc - Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 Advanced Prostate Cancer Consensus Conference (APCCC) Meeting, Lugano, Switzerland, Thurs, Apr 25 - Sat, Apr 27, 2024.
References:
- Freedland SJ, de Almeida Luz M, De Giorgi U, et al. Improved Outcomes with Enzalutamide in Biochemically Recurrent Prostate Cancer. N Engl J Med 2023 Oct 19;389(16):1453-1465.
- Johnson RE, Kermott CA, Murad MH. Gynecomastia – Evaluation and current treatment options. Ther Clin Risk Manag. 2011:7:145-148.


