(UroToday.com) The 2024 Advanced Prostate Cancer Consensus Conference (APCCC) held in Lugano, Switzerland between April 25th and 27th was host to a high-risk and locally advanced prostate cancer session. Dr. Martin Gleave discussed how high-risk and locally advanced prostate cancer should be defined in clinical practice within the context of current diagnostic and therapeutic options.
Dr. Gleave highlighted that there are numerous risk stratification systems currently available:
- Partin Tables: Predict adverse pathologic features following radical prostatectomy
- D’Amico criteria:
- Low, medium, and high-risk based on PSA, clinical T stage, and Gleason score
- High-risk: PSA >20, T stage ≥cT2b, Gleason Score 8 – 10
- Predicts PSA failure
- Low, medium, and high-risk based on PSA, clinical T stage, and Gleason score
- Nomograms, such as Kattan, Briganti, MSKCC, Gandaglia
- Continuous scales that incorporate PSA, Gleason grade, clinical stage, and pathologic features
- Predict 5- and 10-year PSA-free survival following radical prostatectomy
- Consensus
- NCCN
- High-risk: cT3a or Gleason Score 8 – 10 or PSA >20
- Very high-risk: cT3b–4 or multiple risk factors
- RTOG:
- High-risk
- PSA 20 – 100, GS >7, any T stage OR
- PSA <100 ng/ml, GS 8 – 10, cT2; GS 7 + either ≥cT3 or node-positive
- High-risk
- NCCN
Does the definition of high-risk prostate cancer matter? In other words, can we use the different classification systems interchangeably with similar clinical outcomes/prognoses, or does one over/underestimate risk compared to the others? In 2020, Knipper et al. compared seven definitions of high-risk prostate cancer in 26,364 patients treated with radical prostatectomy between 1992 and 2017. Depending on the definition, patients with high-risk prostate cancer comprised between 0.9% (cT3) and 20.3% (D'Amico high-risk) of the population. Ten-year biochemical recurrence-free survival rates varied from 36% (≥cT2c) to 47.4% (National Comprehensive Cancer Network high-risk). Ten-year metastasis-free survival rates varied from 56.6% (Gleason Score 9-10/Grade Group 5) to 77.5% (PSA ≥ 20 ng/ml). Ten-year cancer-specific survival rates varied from 86.6% (cT3) to 94.5% (PSA ≥ 20 ng/ml).1
Dr. Gleave noted that it is important to differentiate between patients with high versus very high-risk disease. In a 2014 retrospective cohort study from Johns Hopkins of 753 men with NCCN high-risk localized prostate cancer, ‘very high-risk’ disease was defined by ≥1 of the following:
- Primary pattern 5
- ≥5 cores with Gleason Score 8 – 10
- Multiple NCCN high-risk features
Compared to patients with high-risk disease, these men with very high-risk disease had increased rates of metastasis (HR: 2.75) and cancer-specific mortality (HR: 3.44, p<0.001).2 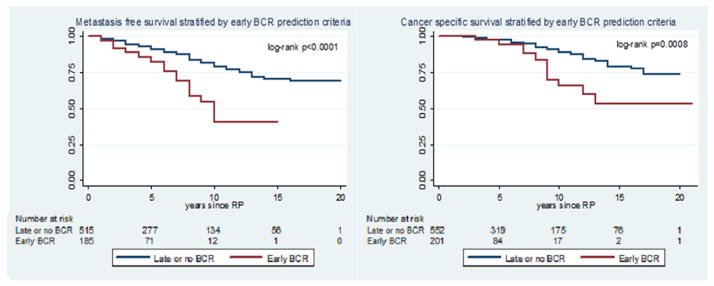
These definitions also have very important implications for clinical trial enrolment/success. One prime example of this is the CALGB 90203 trial of neoadjuvant ADT + docetaxel in localized, high-risk prostate cancer patients. Initially, this trial only allowed for the recruitment of men with a Kattan pre-operative nomogram-predicted probability of freedom from biochemical recurrence 5 years from surgery of <60%, with the investigators projecting that this would account for ~15% of candidates. However, it became clear that only ~5% of patients would be eligible once the trial opened for enrollment, with underwhelming recruitment, and the protocol was amended to enroll men with Gleason Score 8 – 10 or <60% probability of freedom from biochemical recurrence at 5 years post-surgery per the Kattan nomogram.3 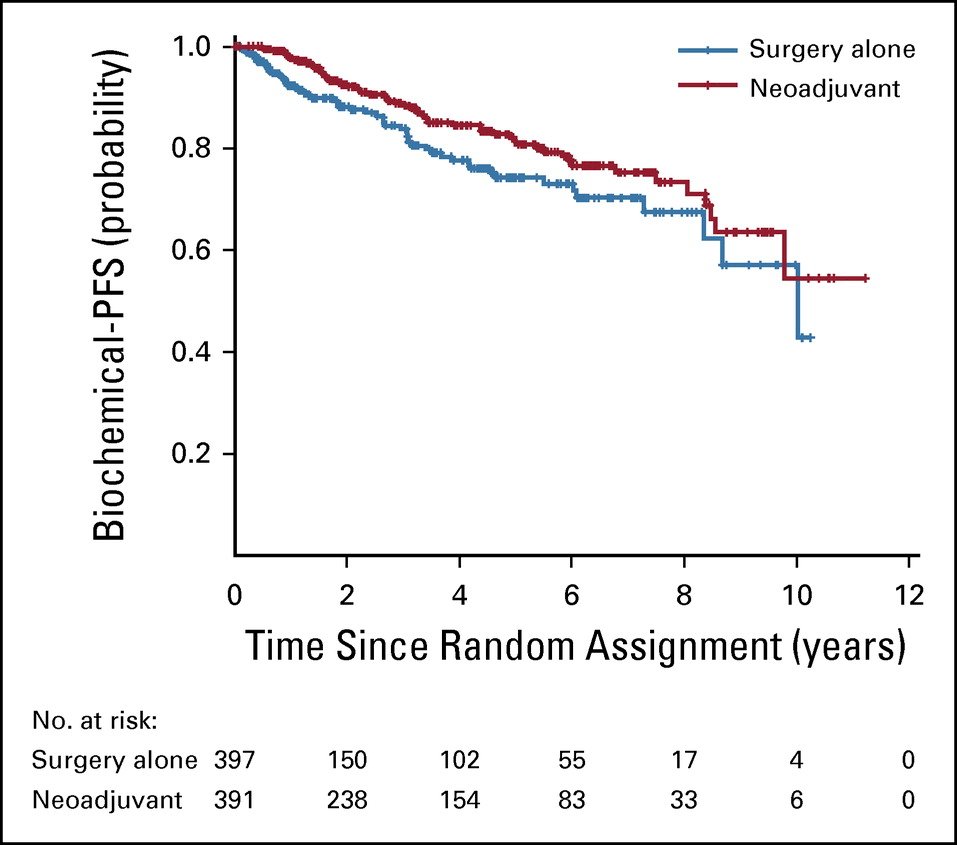
Dr. Gleave noted that the volume of the tumor, as quantified by the number of positive biopsy cores, is an important component of disease risk stratification. For example, a patient with cT3a disease with Gleason score of 8 in 8/12 cores is likely to have worse outcomes compared to a similar patient with only 2/12 cores positive for disease with a similar PSA level. CAPRA ≥6 defines disease extent by percentage of positive cores < or ≥33%, whereas the NCCN incorporates ≤ or >50% core involvement as its disease volume cut-off.
One issue with current risk stratification systems/guidelines/nomograms is that they all incorporate clinical stage. However, Dr. Gleave argued that a digital rectal examination is inaccurate for distinguishing cT2 from T3 and T4 disease, and we need to remove cT status from these systems in favor of volume of positive cores.
The current ongoing challenges in defining prostate cancer risk include the following:
- Prostate cancer is often multifocal/multiclonal in origin, underpinning intra-patient spatial heterogeneity
- Current imaging and biopsy techniques are imperfect and under-sample disease, limiting accuracy of prognostic subgrouping
- Clinical T stage is highly subjective
- Grade-defined risk can be misleading
- What is worse? PSA 11 ng/ml with Gleason score 8 in 1 core or 4+3 in 5 cores or 3+4 in 10 cores?
- How do we weigh systematic versus MRI-targeted cores, particularly when considering disease volume?
- We need to incorporate prostate biopsy core lengths and histologic patterns/variants
- Most important: Presence of pattern 5, volume of pattern 4
- How should we incorporate biomarkers, including somatic signatures and germline testing?
- How will imaging findings be incorporated?
- MRI
- PSMA-PET
Current ongoing trials of neoadjuvant therapy for high-risk patients, such as PROTEUS (ADT +/- apalutamide) and GUNS (Apalutamide + ADT +/- additional genomic biomarker-driven therapy) enrich for patients with high intra-prostate tumor volume and, thus, metastatic recurrence, noting that metastasis-free survival is a co-primary endpoint in such trials. The eligibility criteria for such trials are summarized below:
With regards to genomic biomarkers for risk stratification, currently, the NCCN guidelines note that “tissue-based molecular biomarkers may improve risk stratification when added to standard clinical parameters, but the Expert Panel endorses their use only if likely to affect a clinical decision…Overall, these are not recommended for routine use as not prospectively shown to improve long-term outcomes.”
What is clear however is that appropriate risk stratification is critical for the selection of patients for treatment intensification strategies. The 2022 pooled STAMPEDE data of abiraterone acetate +/- enzalutamide addition to ADT + prostate radiotherapy in very-high risk patients, defined as node-positive disease or node-negative with ≥2 of cT3-4, Gleason sum score of 8–10, and/or PSA ≥40 ng/ml, significantly improved overall survival (HR: 0.60, 95% CI: 0.48–0.73, p<0.0001).4 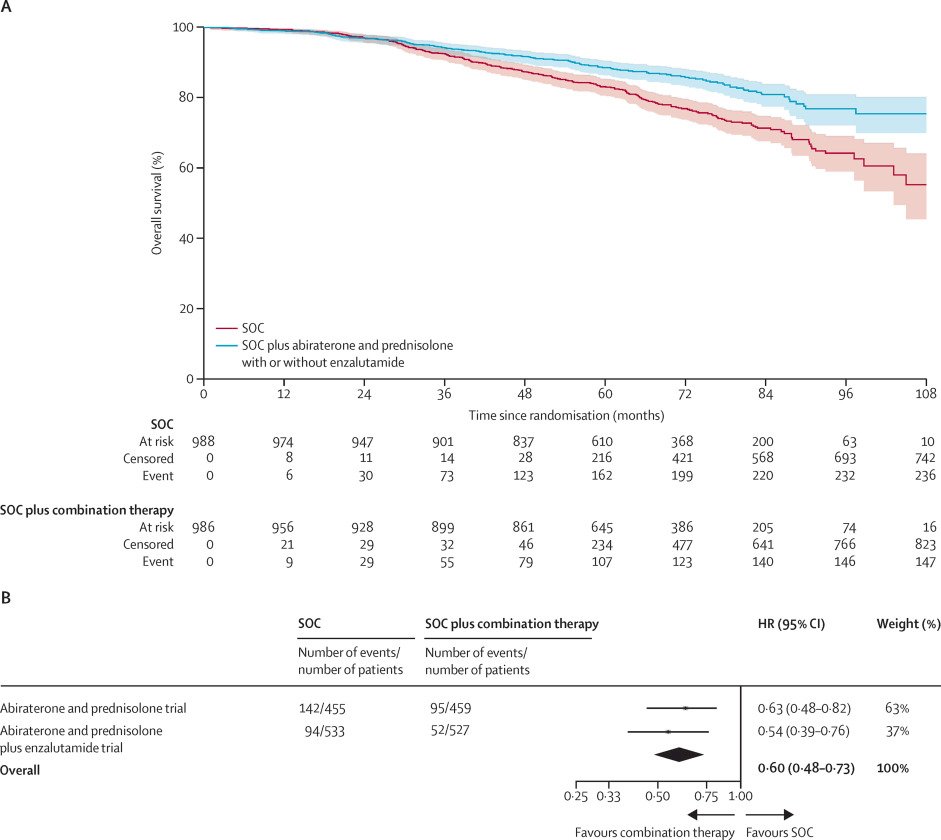
Given the current limitations of risk classification systems, Dr. Gleave proposed the following schema for defining high-risk, localized prostate cancer, which incorporates PSMA PET/CT findings (noting that N1-2 and M1a disease on PSMA PET/CT can still be considered ‘localized’ given that many patients historically with negative conventional imaging and high-risk features may have had positive PSMA-PET/CTs), disease volume (number of cores), and genomic test results: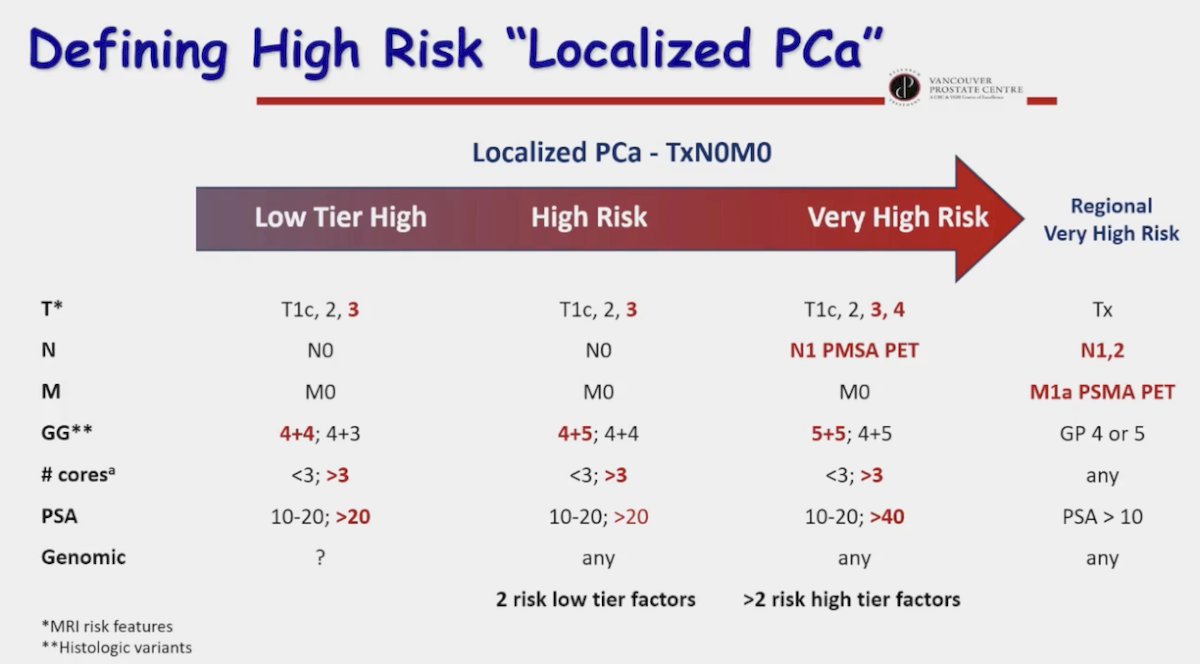
Presented by: Martin Gleave, MD, FRCSC, FACS, Distinguished Professor and Head, Department of Urologic Sciences, University of British Columbia, Vancouver, BC
Written by: Rashid Sayyid, MD, MSc - Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 Advanced Prostate Cancer Consensus Conference, Lugano, Switzerland, April 25th - April 27th, 2024
References:
- Knipper S, Karakiewicz PI, Heinze A, et al. Definition of high-risk prostate cancer impacts oncological outcomes after radical prostatectomy. Urol Oncol. 2020;38(4): 184-90.
- Sundi D, Wang V, Pierorazio PM, et al. Identification of men with the highest risk of early disease recurrence after radical prostatectomy. Prostate. 2014;74(6): 628-36.
- Eastham JA, Heller G, Halabi S, et al. Cancer and Leukemia Group B 90203 (Alliance): Radical Prostatectomy With or Without Neoadjuvant Chemohormonal Therapy in Localized, High-Risk Prostate Cancer. J Clin Oncol. 2020;38(26): 3042-50.
- Attard G, Murphy L, Clarke NW, et al. Abiraterone acetate and prednisolone with or without enzalutamide for high-risk non-metastatic prostate cancer: a meta-analysis of primary results from two randomised controlled phase 3 trials of the STAMPEDE platform protocol. The Lancet. 2022;399(10323): 447-60.


