(UroToday.com) The 2024 Advanced Prostate Cancer Consensus Conference (APCCC) held in Lugano, Switzerland was host to a high-risk and locally advanced prostate cancer session. Dr. Piet Ost debated in favor of pelvic radiotherapy, as part of a multimodal regimen, as the best approach to manage a fit patient with high-risk, localized, or locally advanced prostate cancer.
Dr. Ost noted that there are a multitude of factors that need to be considered when risk stratifying these patients as potentially having high/very high-risk or locally advanced disease:
- PSA >20 ng/ml
- Gleason score 8–10
- Stage cT3–4 and/or N1

And while patients with organ confined disease remain candidates for local therapy, as their risk increases from low-intermediate to high-very high, particularly in the castrate-sensitive setting, the role of systemic therapy becomes more relevant for improved disease control. As such, there is a clear role for multimodal treatment approaches (i.e., combined local and systemic therapy) for these patients.
It has long been established that the doublet combination of radiotherapy plus ADT, compared to radiotherapy alone, improves survival outcomes for patients with high-risk prostate cancer. Questions regarding the optimal dose of radiotherapy and duration of ADT have persisted. In an individual patient-data network meta-analysis from the MARCAP consortium, Kishan et al. demonstrated that the greatest improvement in outcomes was observed with long-term ADT, irrespective of radiotherapy dose, followed by addition of short-term ADT, again irrespective of radiotherapy dose. Notably, radiotherapy dose escalation (64 to <74 Gy versus ≥74 Gy) did not improve metastasis-free survival irrespective of whether long-term, short-term, or no ADT was concurrently administered, although radiotherapy dose escalation did improve biochemical recurrence-free survival.1 These results suggest that the issue at hand here is not one of inadequate local control with radiotherapy, but of distant disease failure/recurrences among those patients with ‘bad biology’.
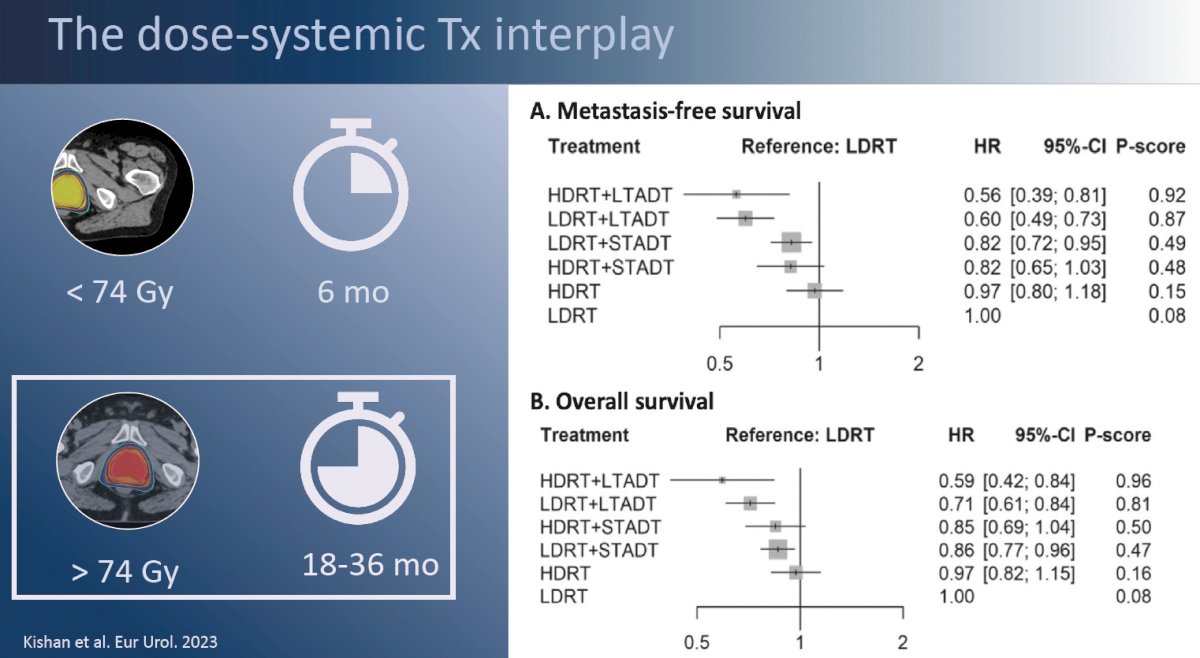
This point is further emphasized by the results of the recently presented GETUG-AFU 18 randomized trial of dose-escalated radiotherapy (80 Gy versus 70 Gy) combined with long-term ADT in high-risk prostate cancer patients. Dose escalation from 70 to 80 Gy improved 10-year prostate cancer-specific survival from 90% to 96% in these patients with one high-risk feature. Conversely, in the pooled STAMPEDE data of systemic therapy intensification with addition of abiraterone acetate +/- enzalutamide to prostate radiotherapy (74 Gy in 37 fractions) +ADT, patients with very high-risk features (i.e., ≥2 risk factors) had an 8-year overall survival of 62%. While across trial comparisons are fraught with numerous limitations, these results strongly suggest that the patient’s underlying disease risk profile/biology, and thus subsequent risk of distant disease spread/failure, is the major driver of these patients’ outcomes.
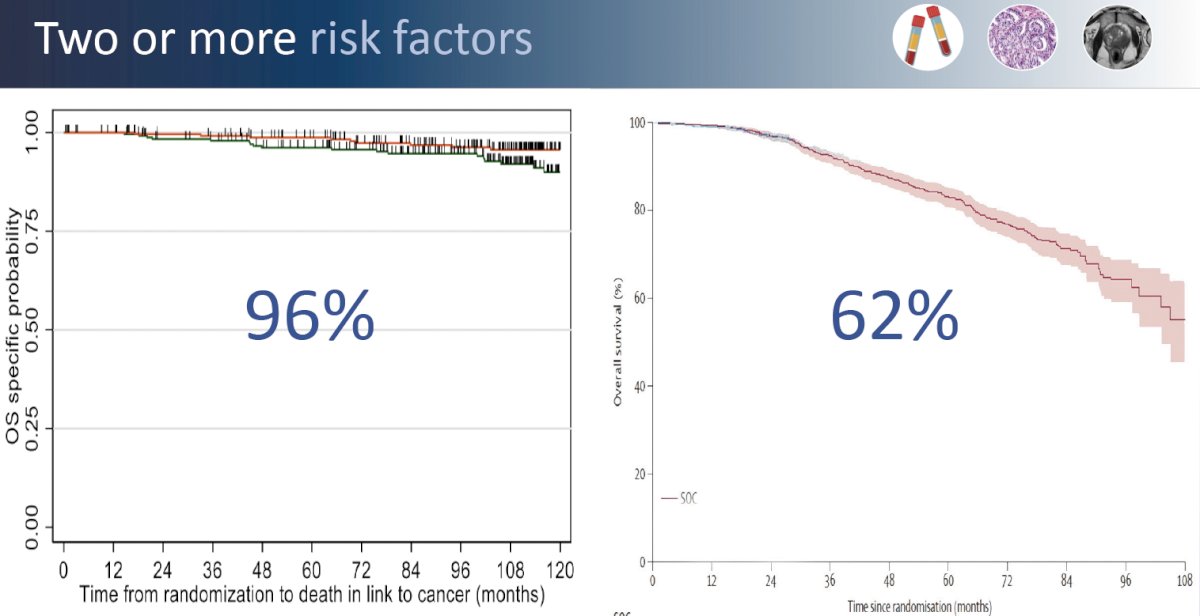
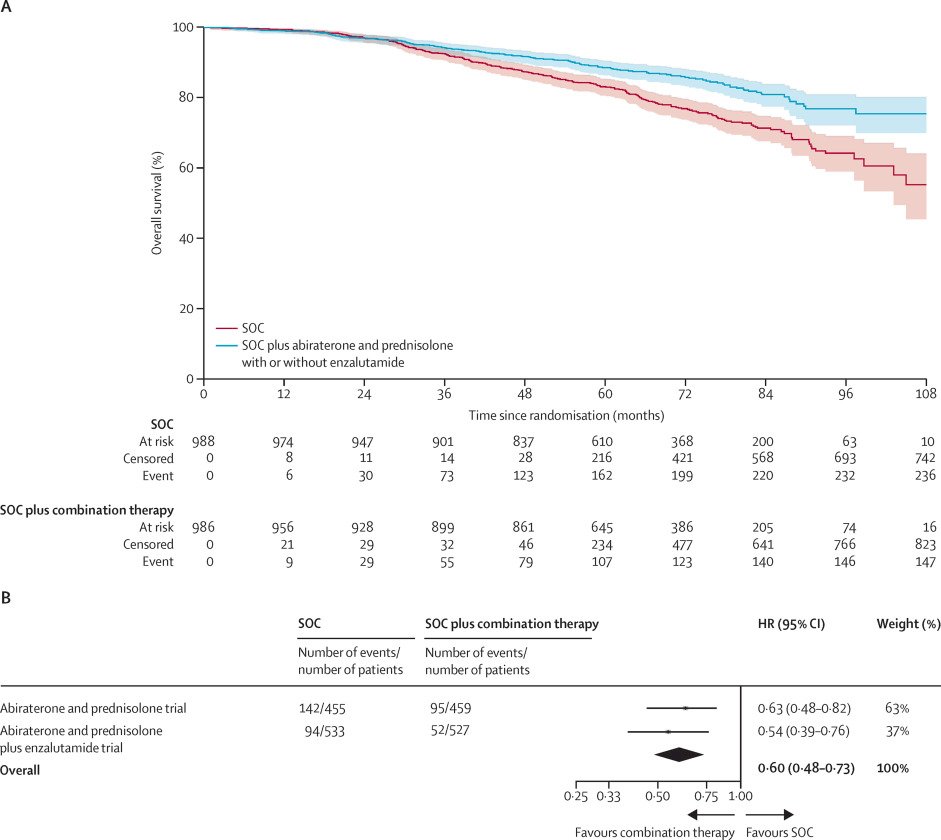
This emphasizes the importance of systemic therapy intensification for such patients at an elevated risk of systemic disease spread. The addition of abiraterone acetate +/- enzalutamide to prostate radiotherapy + ADT in patients with node positive disease or node negative with ≥2 of cT3-4, Gleason sum score of 8–10, and/or PSA ≥40 ng/ml significantly improved overall survival (HR: 0.60, 95% CI: 0.48–0.73, p<0.0001).2
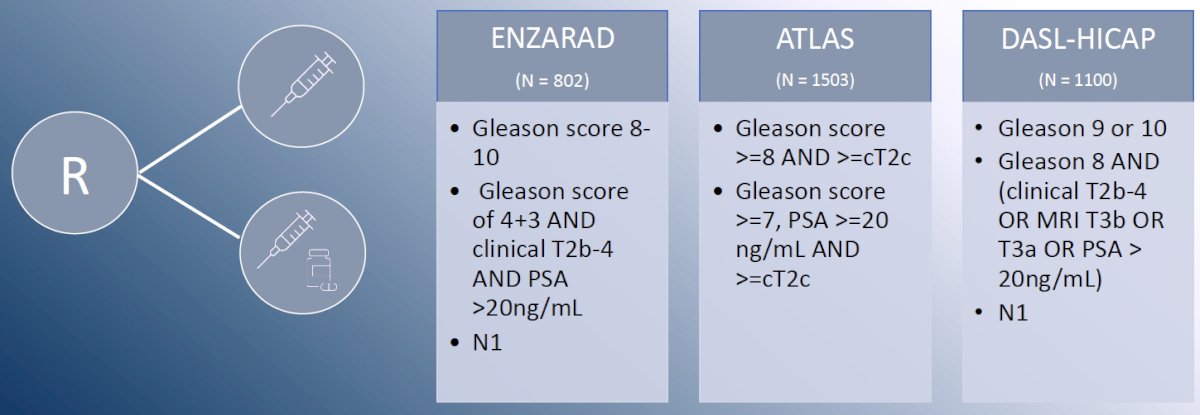
Numerous ongoing trials are evaluating systemic therapy intensification with addition of an androgen receptor pathway inhibitor to radiotherapy + ADT in patients at high-risk of systemic failure: ENZARAD (enzalutamide), ATLAS (apalutamide), and DASL-HICAP (darolutamide).
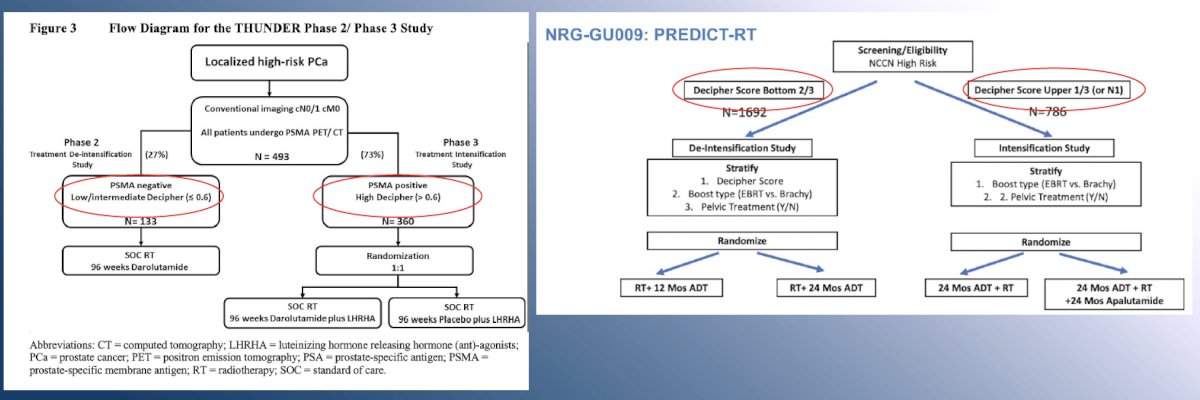
In addition to clinical risk factors, ongoing trials in the localized, high-risk disease space are incorporating the use of molecular imaging (PSMA-PET/CT) and/or Decipher® score to facilitate risk-adapted treatment intensification/de-intensification strategies in patients scheduled for radiotherapy:
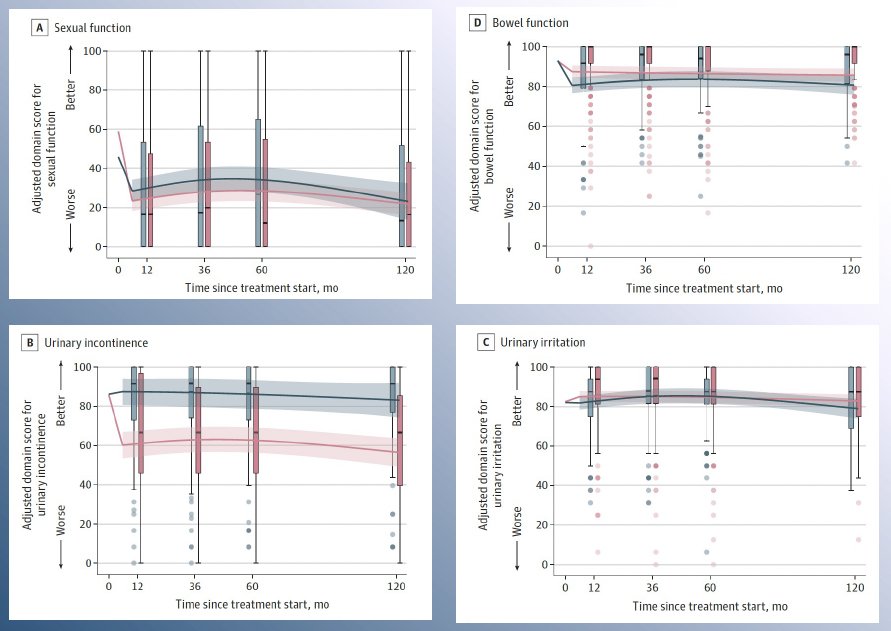
What are the treatment ‘variables’ in this multimodal approach? Can we substitute surgery for radiotherapy for example? Dr. Ost noted that quality of life remains an important consideration when choosing the local treatment modality for these patients. In a 2024 SEER analysis of 2,445 patients with localized prostate cancer, Al Awamlh et al. demonstrated that radical prostatectomy was associated with worse urinary incontinence at 10-year follow-up, compared with radiotherapy or surveillance among patients with a favorable prognosis. No significant differences in sexual function between radiotherapy and radical prostatectomy were observed, and among men with unfavorable-prognosis disease, external beam radiotherapy plus ADT was associated with worse bowel and hormone function at 10-year follow-up, compared with radical prostatectomy.4 As such, clinicians need to consider all such variables and educate patients to allow for a personalized, informed decision-making process.
Additionally, when offering local therapy to patients, it is important to consider the patient-level variables that are associated with an increased risk of failure with that treatment modality. With regards to radical prostatectomy, patients with a serum PSA concentration >10 ng/ml, ≥cT2b, Gleason score 9–10 at biopsy, increasing number of cores with high-grade cancer, and >50% core involvement are at increased risk of biochemical recurrence post-operatively.4 Given that patients with high-risk disease harbor ≥1 such variable, this suggests that radical prostatectomy monotherapy is not advised for high-risk patients.4
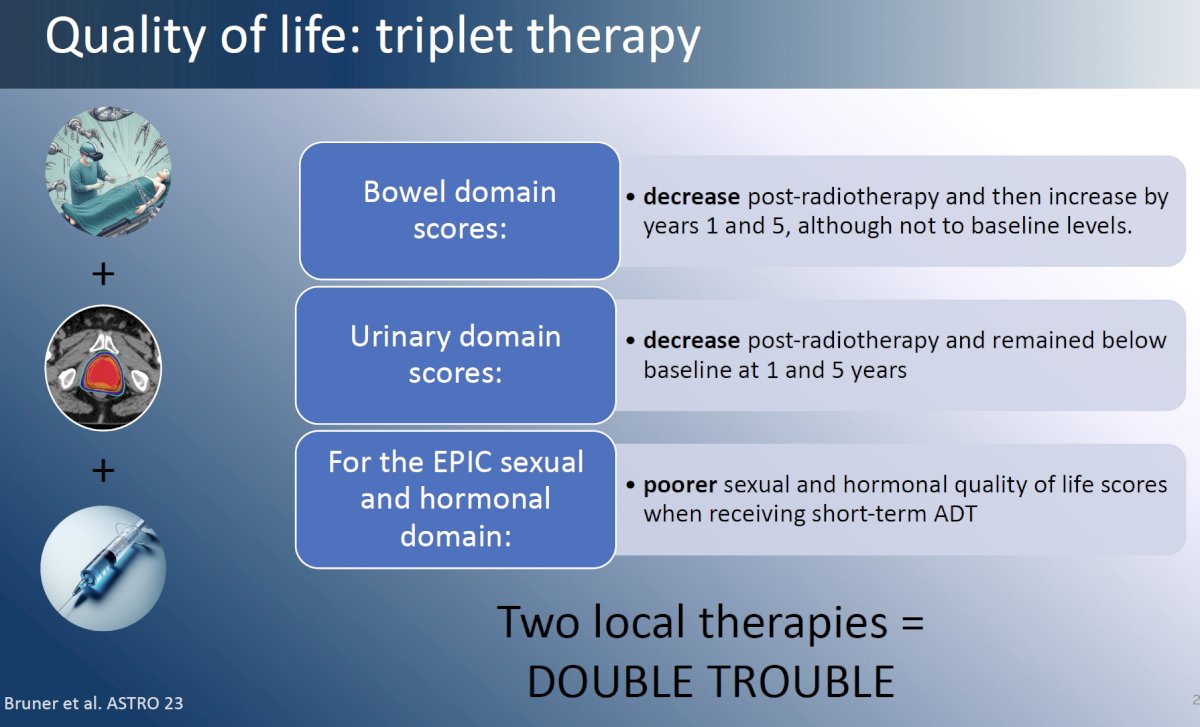
What about triplet therapy with surgery + radiotherapy + hormone therapy? To date, there is no proven benefit to such triplet therapy, with the current literature suggesting that early salvage radiotherapy is non-inferior to adjuvant radiotherapy in the majority of patients.5-7 Additionally, there is clear evidence that when both local therapies are concurrently administered, there is significant, cumulative worsening of bowel, urinary, and sexual function domain scores.
Based on the current evidence, Dr. Ost concluded that:
- As the number of high-risk prostate cancer features increases, so does the benefit of systemic therapy for these patients.
- Doublet therapy with radiotherapy + ADT offers excellent outcomes for high to very high-risk prostate cancer patients.
- Triplet therapy increases morbidity over doublet therapy, without a proven benefit.
Presented by: Piet Ost, MD, PhD, Associate Professor, Radiation Oncology, Faculty of Medicine and Health Sciences, University of Gent, Belgium
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 Advanced Prostate Cancer Consensus Conference, Lugano, Switzerland, April 25th – April 27th, 2024
References:
- Kishan AU, Wang X, Sun Y, et al. High-dose Radiotherapy or Androgen Deprivation Therapy (HEAT) as Treatment Intensification for Localized Prostate Cancer: An Individual Patient-data Network Meta-analysis from the MARCAP Consortium. Eur Urol. 2022;82(1): 106-114.
- Attard G, Murphy L, Clarke NW, et al. Abiraterone acetate and prednisolone with or without enzalutamide for high-risk non-metastatic prostate cancer: A meta-analysis of primary results from two randomized controlled phase 3 trials of the STAMPEDE platform protocol. Lancet 2022;399(10323):447-460.
- Al Awamlh B, Wallis CJD, Penson DF, et al. Functional Outcomes After Localized Prostate Cancer Treatment. JAMA. 2024;331(4): 302-317.
- Pierorazio PM, Ross AE, Lin BM, et al. Preoperative characteristics of high-Gleason disease predictive of favourable pathological and clinical outcomes at radical prostatectomy. BJU Int. 2012;110(8): 1122-8.
- Parker CC, Clarke NW, Cook AD, et al. Timing of radiotherapy after radical prostatectomy (RADICALS-RT): A randomized, controlled phase 3 trial. Lancet 2020;396(10260):1413-1421.
- Sargos P, Chabaud S, Latorzeff I, et al. Adjuvant radiotherapy versus early salvage radiotherapy plus short-term androgen deprivation therapy in men with localized prostate cancer after radical prostatectomy (GETUG-AFU 17): A randomized, phase 3 trial. Lancet Oncol 2020;21(10):1341-1352.
- Kneebone A, Fraser-Browne C, Duchesne GM, et al. Adjuvant radiotherapy versus early salvage radiotherapy following radical prostatectomy (TROG 08.03/ANZUP RAVES): A randomized, controlled, phase 3, non-inferiority trial. Lancet Oncol. 2020;21(10):1331-1340.


