(UroToday.com) The 2024 Advanced Prostate Cancer Consensus Conference (APCCC) held in Lugano, Switzerland was host to a high-risk and locally advanced prostate cancer session. Dr. Jochen Walz discussed the current standard for regional lymph node staging in patients with high-risk and locally advanced prostate cancer.
Currently, international guidelines, such as those of the EAU/ESTRO, strongly recommend performing an extended pelvic lymph node dissection prior to radical prostatectomy in patients with locally advanced prostate cancer.
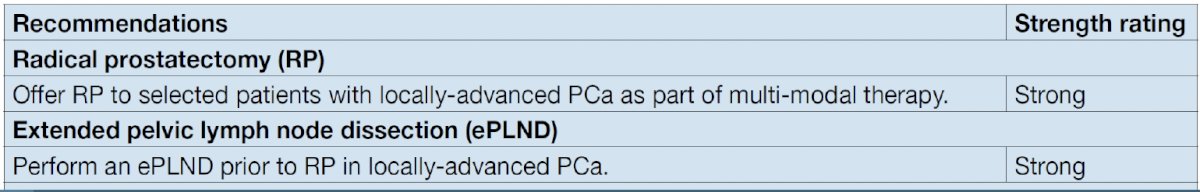
While there are numerous pelvic lymph node dissection templates described, the gold standard remains an extended pelvic nodal dissection. Among prostate cancer patients who undergo an extended template dissection, 94% of patients are correctly staged and 87% of positive nodes are removed. However, this does mean that 13% of positive nodes are missed with such templates. This false negative rate can be reduced by adding presacral lymph nodes to this template.1
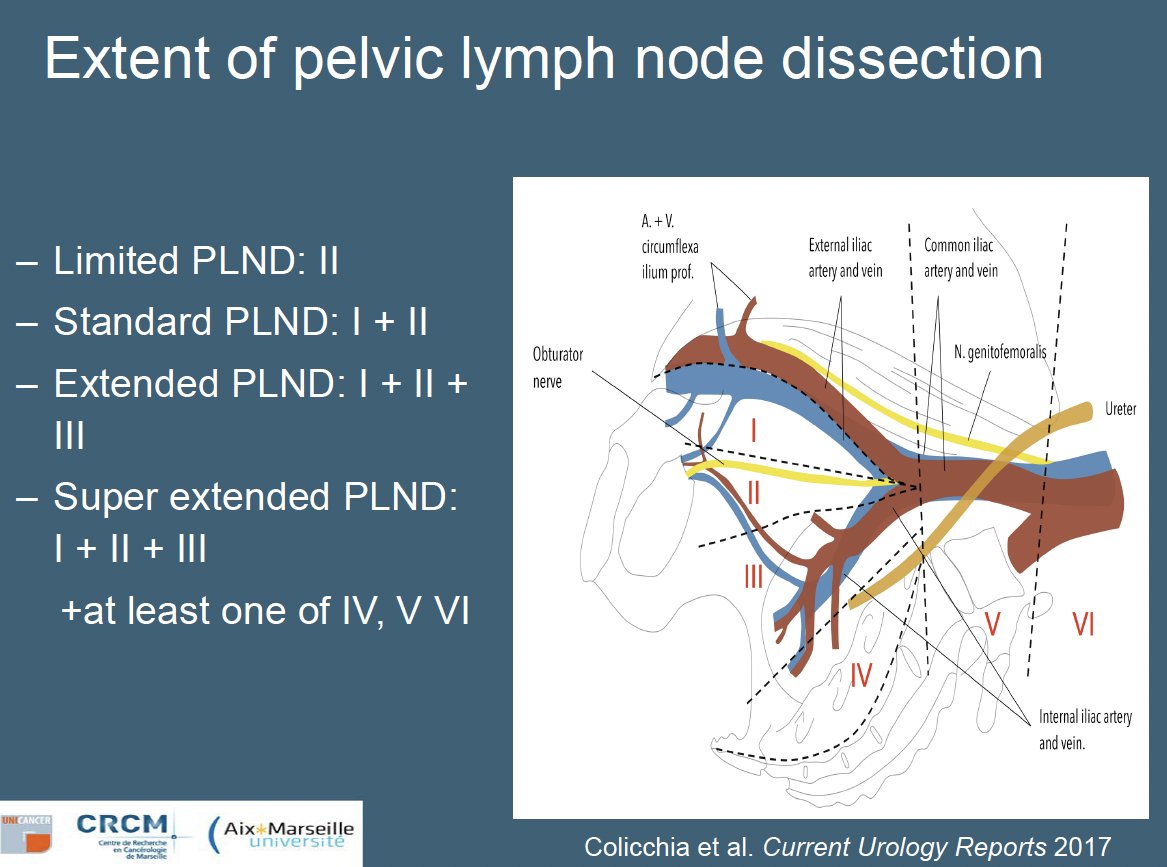
How does the yield from an extended lymph node template compare to that from a limited pelvic nodal dissection? In 2021, Lestingi et al. published the results of a prospective phase 3 trial in 300 patients with clinically localized intermediate- to high-risk prostate cancer who were randomized 1:1 to either a limited pelvic lymph node dissection (obturator nodes) or an extended template (obturator, external iliac, internal iliac, common iliac, and presacral nodes). While there was no significant difference in biochemical recurrence-free survival between the two arms, patients in the extended template were more likely to have pN1 disease detected (17% versus 3.4%).2 As such, Dr. Walz argued that extended lymph node dissection remains the most reliable template given its superior sensitivity.2
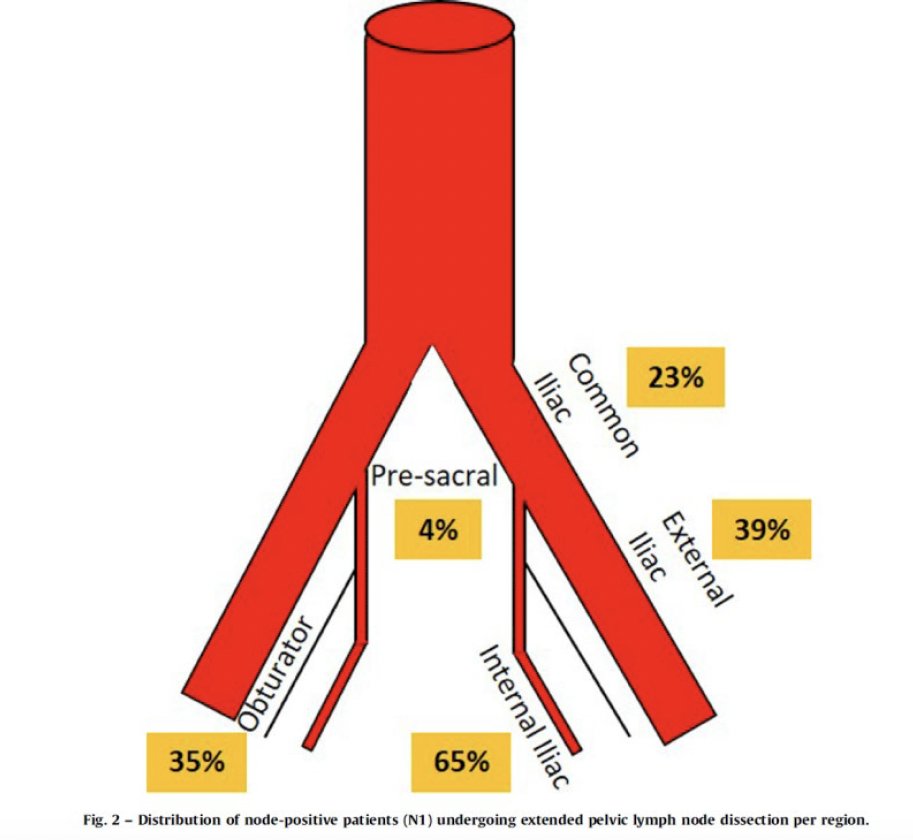
With the approval of PSMA-PET/CT for the initial staging of intermediate- and high-risk prostate cancer patients being considered for definitive local therapy, the ‘hope’ is that this test will be able to rule out patients without pathologic nodal involvement, and thus forgo a pelvic lymph node dissection in these patients. Published in 2020, proPSMA is a multi-center, two-arm randomized controlled trial of men with histologically confirmed prostate cancer who were being considered for curative intent radical prostatectomy or radiotherapy. To be eligible for inclusion, men must have had ≥1 high-risk factor including PSA ≥ 20 ng/mL, ISUP grade group 3-5, or clinical stage T3 or greater. Following enrollment, patients were randomly assigned in a 1:1 ratio to either conventional imaging consisting of bone scan and CT or 68Ga-PSMA-11 PET/CT. With regards to the detection of pelvic nodal disease, PSMA PET-CT was significantly more sensitive than conventional imaging for detection of positive nodal disease. Notably, this correlates with an enhanced negative predictive value (i.e., the ability to rule out absence of disease) of 95% versus 77% with conventional imaging.3
Other studies have also assessed the performance of PSMA for lymph node staging. In 2021, Hope et al. published results of a multicenter, single-arm, open-label phase 3 trial evaluating 68Ga-PSMA-11 PET/CT for the detection of pelvic nodal metastases in patients with intermediate and high-risk prostate cancer (at least one of the following: PSA >10 ng/mL, ≥cT2b, Gleason Score ≥7). The sensitivity of PSMA-PET/CT was 40% and the negative predictive value was 81%.4
In the same year, van Kalmthout et al. evaluated the diagnostic performance of 68Ga-PSMA-11 PET/CT in patients with newly diagnosed prostate cancer who had a negative bone scan and a greater than 10% risk for lymph node involvement (Memoria Sloan Kettering Cancer Centre nomogram) prior to planned radical prostatectomy with extended pelvic lymph node dissection. This single arm trial included 103 patients, of whom 97 underwent extended pelvic lymph node dissection (chosen reference standard). In this trial, the sensitivity of PSMA-PET/CT was 41.5% and the negative predictive value was 68%.5
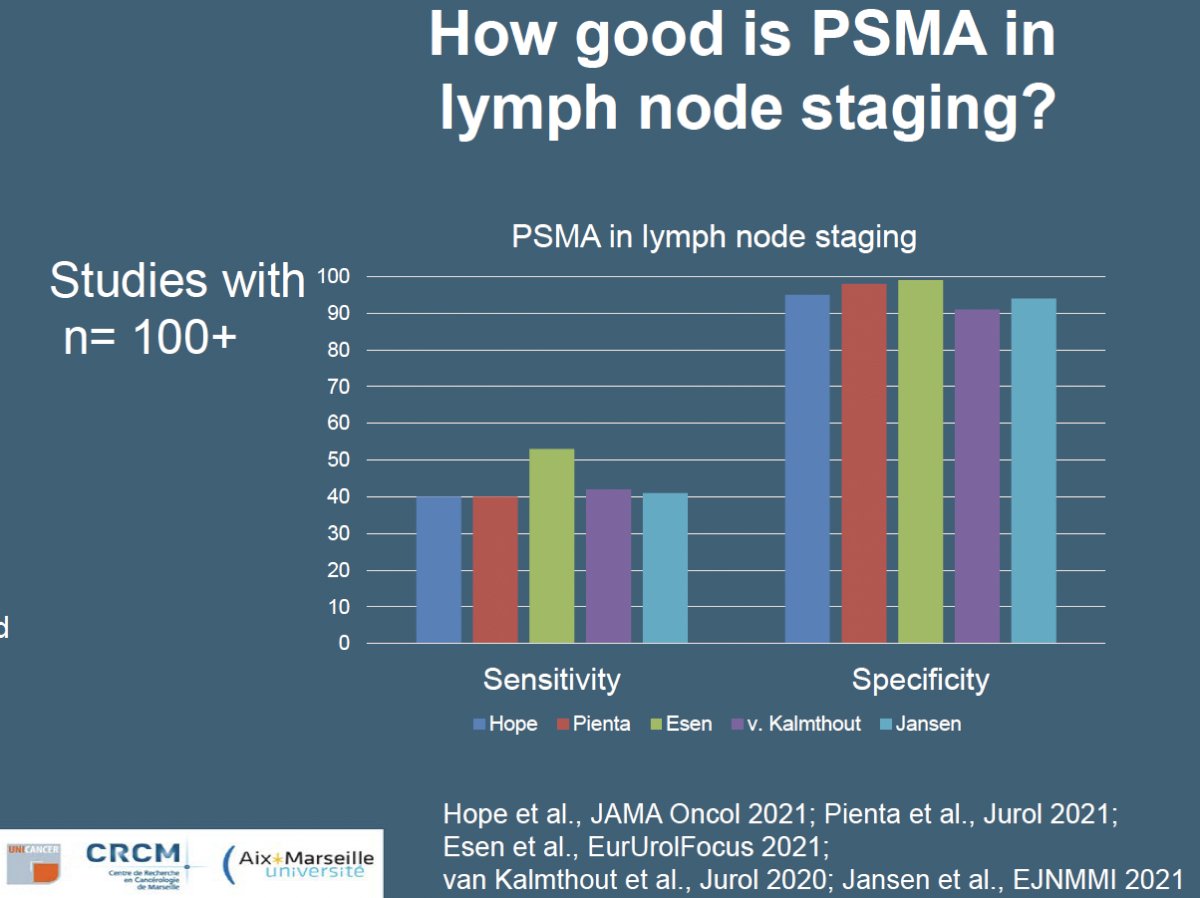
One of the major limitations of PSMA-PET/CT is that it misses those lymph node deposits that are <5 mm in size. The SALT trial, published in 2021, was a prospective multicenter trial that evaluated the diagnostic performance of 18F-DCFPyL PSMA PET/CT for staging high-risk prostate cancer patients prior to treatment. This trial included 117 patients planned for robotic radical prostatectomy with extended pelvic lymph node dissection who had an ≥ 8% risk of lymph node metastases per the Memorial Sloan Kettering Cancer Centre (MSKCC) risk nomogram. In this setting, PSMA-PET/CT had a sensitivity of 41% and a negative predictive value of 90%. Significantly, the median tumor size of PET-detected lymph nodes was 5.5 mm, compared to 1.5 mm for those that were not detected on imaging.6
An additional advantage of PSMA-PET/CT in the initial staging work-up of intermediate/high-risk patients is that it can provide prognostic information. Biochemical recurrence-free and therapy-free survivals are worst for patients with PSMA PET positive lymph nodes, followed by pN1 patients without PSMA PET positive lymph node and then patients without evidence of lymph node metastasis on histology and PSMA PET.7
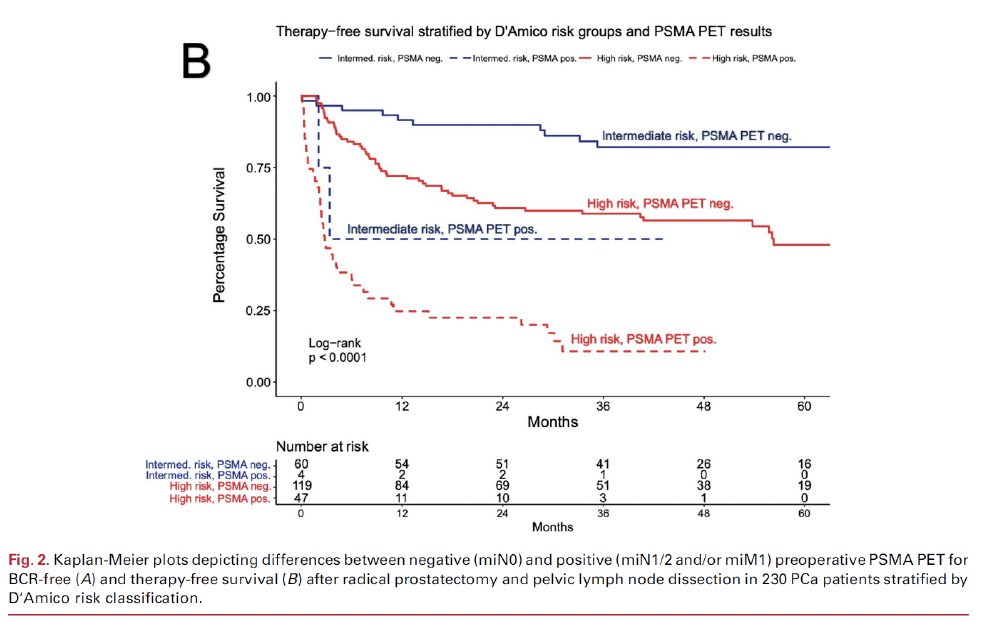
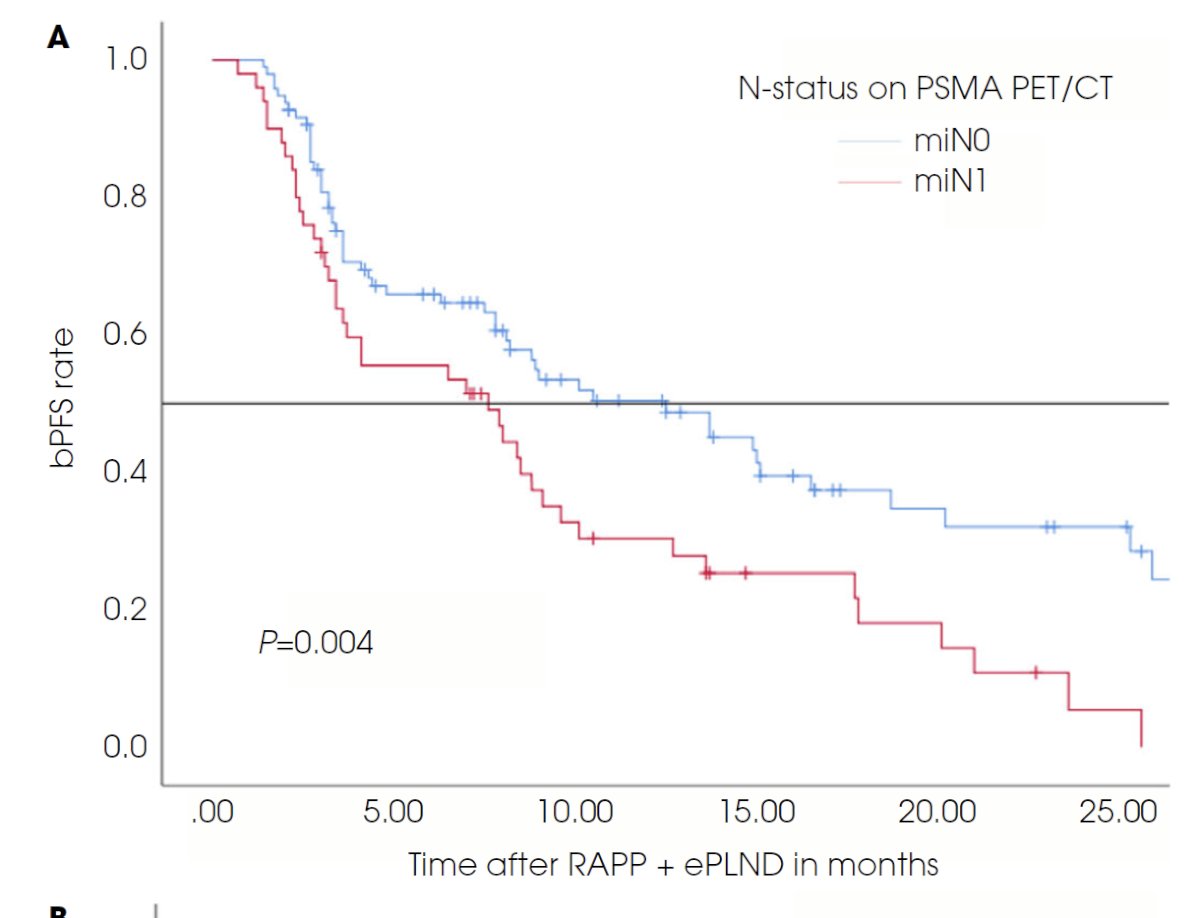
These findings have been corroborated by Meijer et al. who demonstrated that presence of miN1 disease on PSMA PET predicted early biochemical progression following a robotic radical prostatectomy.8
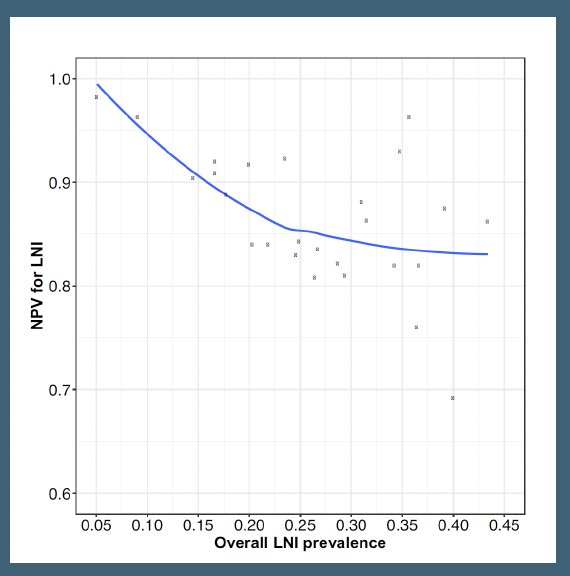
However, to date, it does not appear that PSMA-PET/CT can be reliably used to exclude high-risk patients without pelvic nodal disease and to determine which high-risk patients should or should not undergo a pelvic lymph node dissection. A 2022 systematic review and meta-analysis demonstrated that the sensitivity and negative predictive values were 51% and 81%, respectively, with the negative predictive value decreasing from 99% to 84% as the prevalence of lymph node invasion increased from 5% to 40%.9
Do pelvic lymph node dissections have a therapeutic benefit or are they simply a staging procedure? In the aforementioned phase 3 trial by Lestingi et al., patients with Grade Group 3 to 5 disease undergoing an extended nodal dissection had superior biochemical recurrence-free survival compared to patients undergoing a limited dissection.2
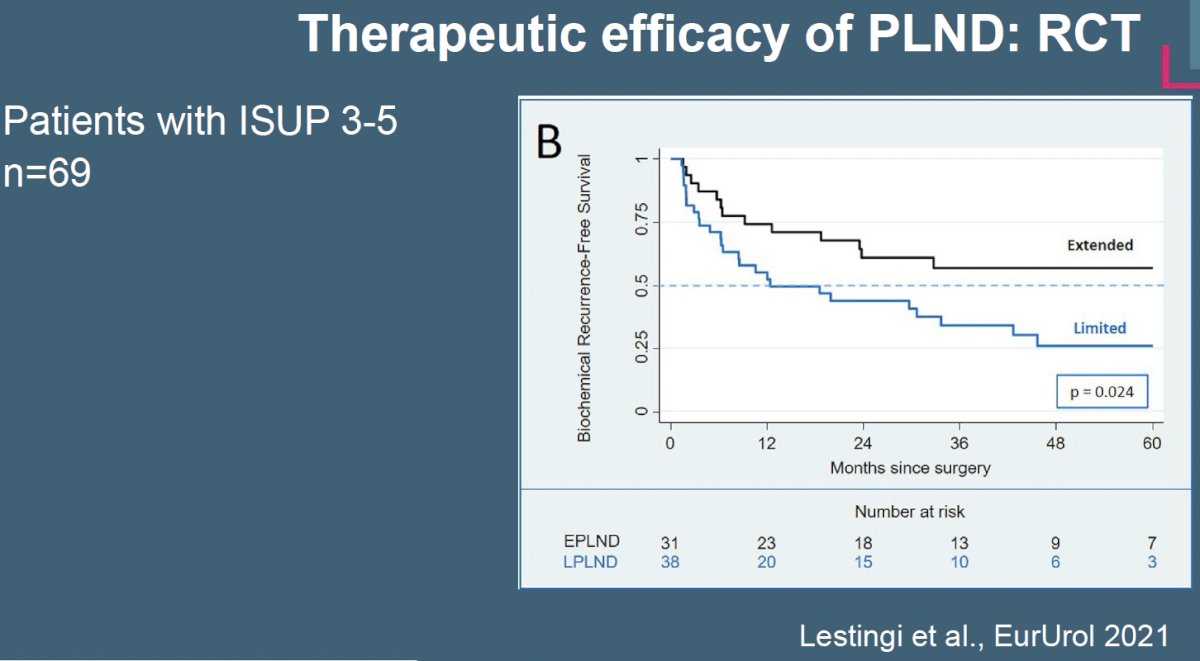
Recently, at EAU 2024, Dr. Karim Touijer presented an update on the MSKCC limited versus extended lymph node dissection randomized clinical trial. Initial results of this trial (n=1,440) have previously been published in European Urology Oncology in 2021 and demonstrated that at a median follow-up of 3.1 years, there was no significant difference in the rate of biochemical recurrence between patients undergoing an extended versus limited pelvic lymph node dissection.10
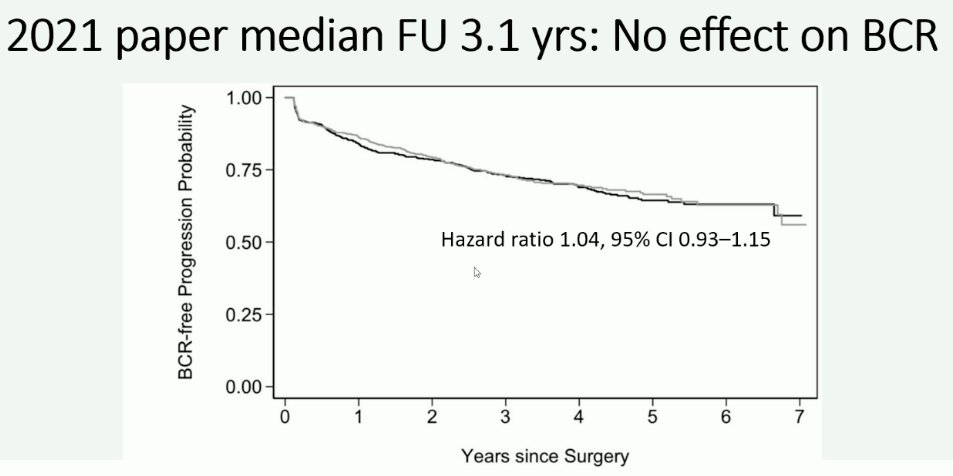
In this trial, a limited lymph node dissection included the external iliac nodes, whereas the extended template additionally included the obturator fossa and hypogastric nodes. Notably, the median lymph node yield was 12 (IQR: 8–17) in the limited group and 14 (IQR: 10–20) in the extended group.
Extended follow-up of this trial, with 4.2 years of follow-up, still demonstrates that there is no significant benefit with extended nodal dissection for biochemical recurrence:
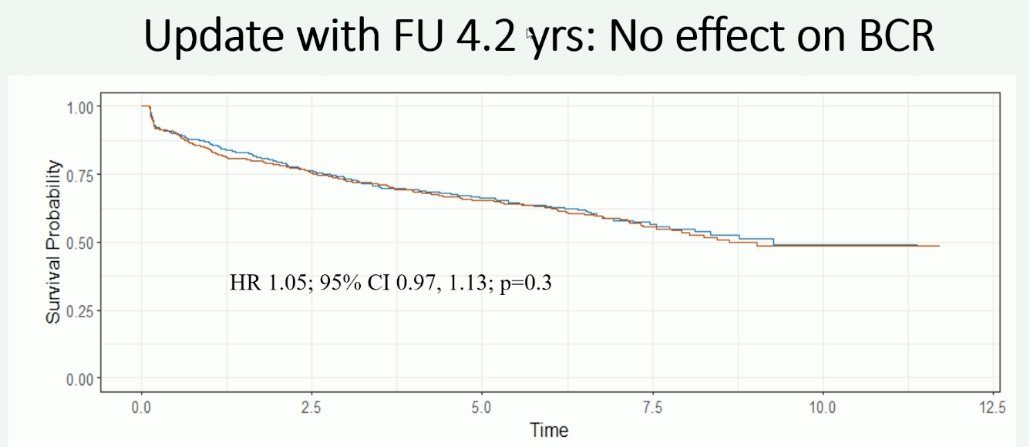
However, patients undergoing an extended lymph node dissection had a reduced incidence of metastases (HR: 0.82, 95% CI: 0.71–0.93, p=0.003).
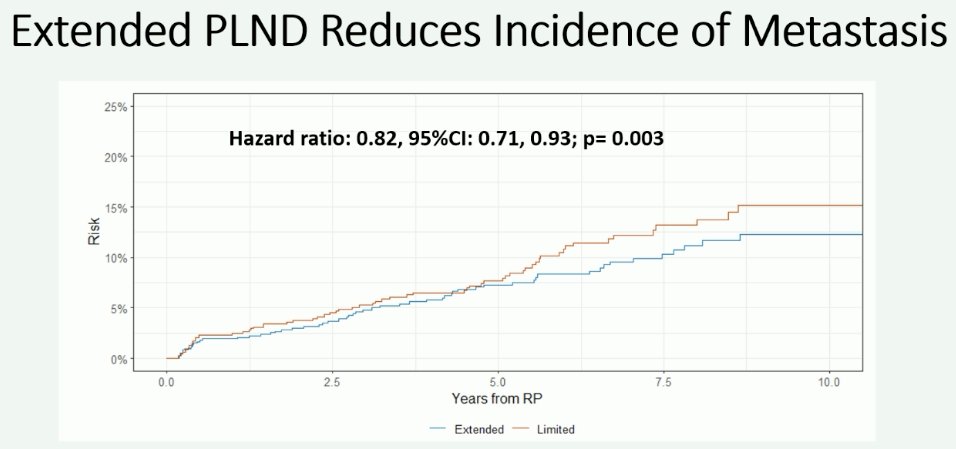
This measure of effect is even more pronounced when analysis is limited to the outcome of distant metastases (HR: 0.75, 95% CI: 0.64–0.88, p<0.001).
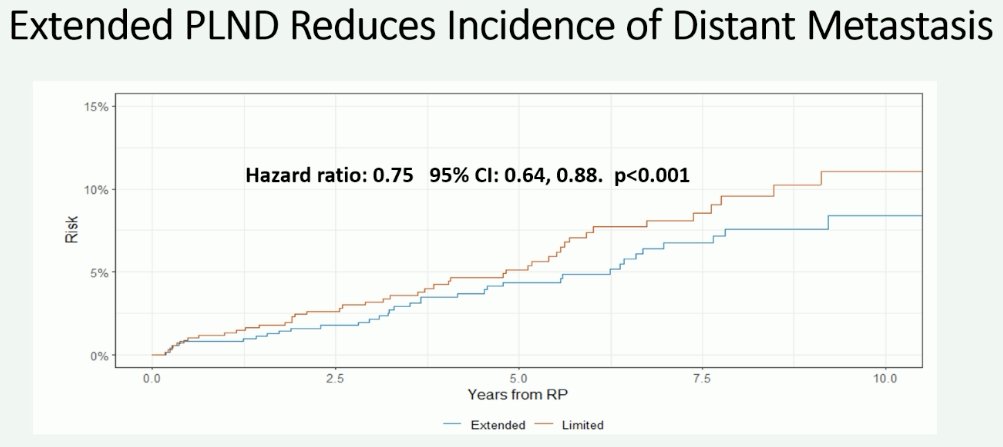
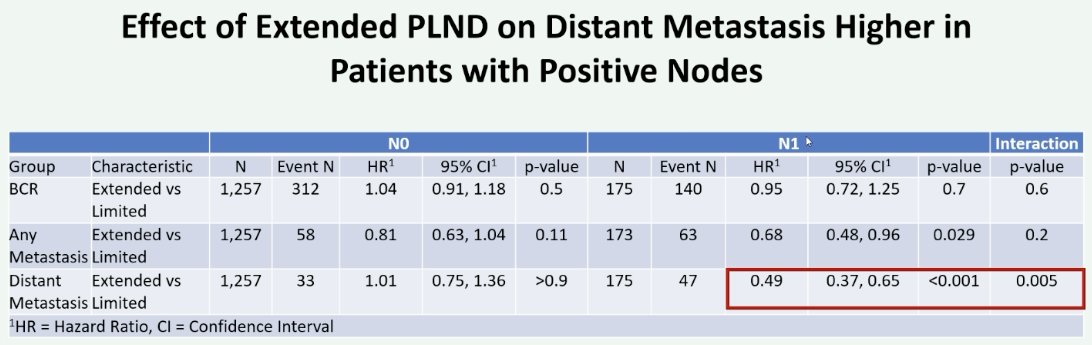
Notably, the greatest distant metastasis-free survival benefit effect appears to be present in patients with pathologic node-positive disease (HR: 0.49, 95% CI: 0.37–0.65, p<0.001).
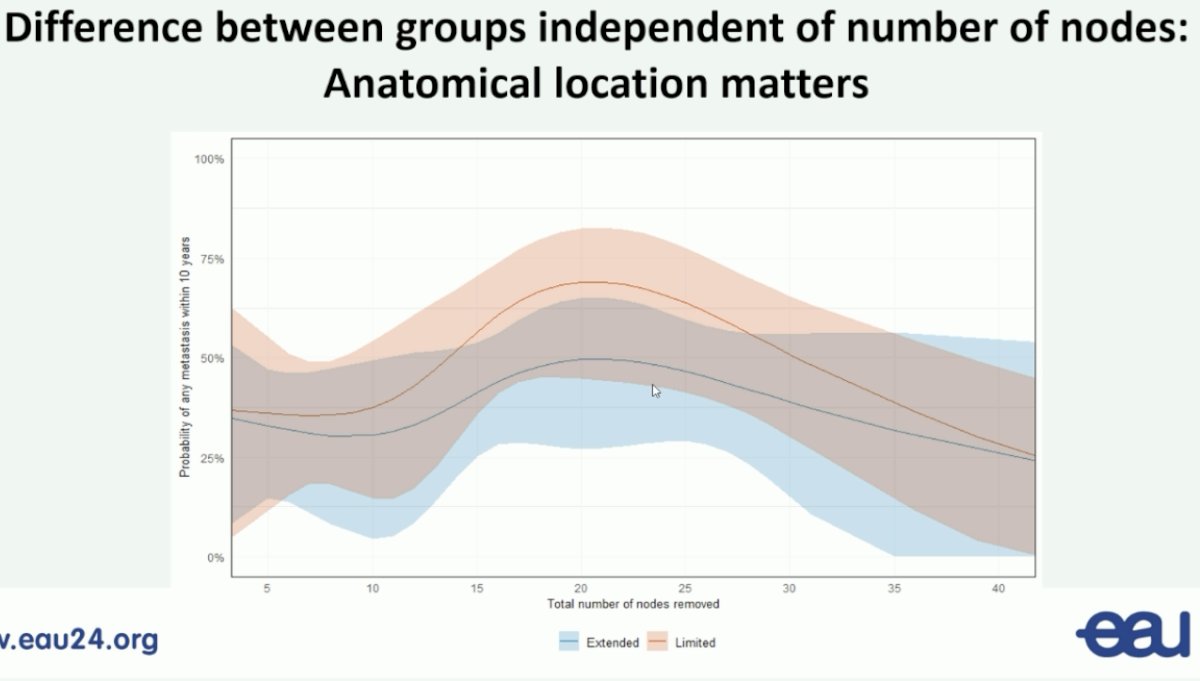
One of the major criticisms/limitations of this trial has been the minimal differences in the nodal yield count between the two arms (medians: 12 versus 14). Analysis by the number of nodes sampled demonstrated that the distant metastasis-free survival benefits of an extended nodal dissection are independent of the number of nodes sampled.
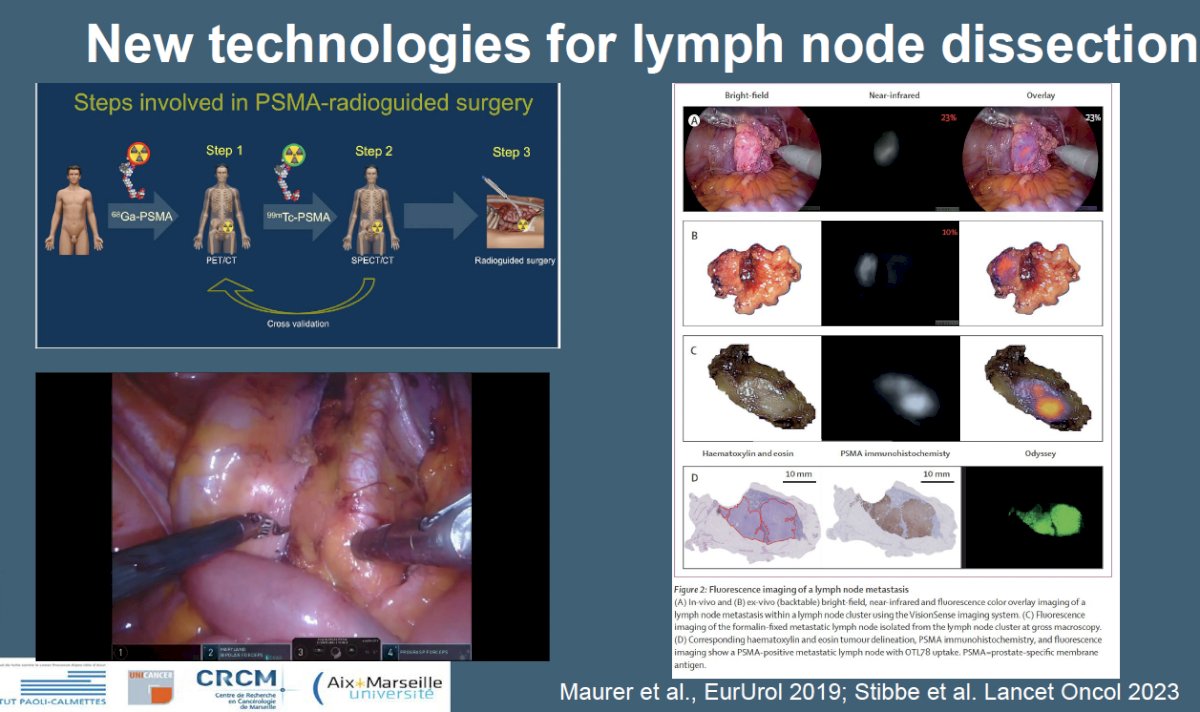
Numerous ongoing trials are evaluating PSMA for lymph node staging, including the Dutch National Randomized Study (NCT05000827): PSMA-PET/CT as a Triage Tool for Pelvic Lymph Node Dissection in Prostatectomy Patients.
Additional new technologies for lymph node dissection incorporating PSMA-radio guided techniques are being developed and soon to be evaluated in multi-institutional phase II trials.
Dr. Walz concluded with his thoughts on what should be the standard for lymph node dissection for prostate cancer patients:
- The standard pelvic lymph node template should be an extended pelvic lymph node dissection.
- It remains unclear if PSMA allows us to forgo a pelvic nodal dissection when negative.
- Extended pelvic lymph node dissection is likely to improve ‘harder’ endpoints such as metastasis-free survival.
Presented by: Jochen Walz, MD, Associate Professor in Urology and Head of the Department of Urology at the Institut Paoli-Calmettes Cancer Centre. Marseille, France
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 Advanced Prostate Cancer Consensus Conference, Lugano, Switzerland, April 25th – April 27th, 2024
References:
- Joniau S, van den Bergh L, Lerut E, et al. Mapping of pelvic lymph node metastases in prostate cancer. Eur Urol. 2013;63(3): 450-8.
- Lestingi JFP, Guglielmetti GB, Trinh Q, et al. Extended Versus Limited Pelvic Lymph Node Dissection During Radical Prostatectomy for Intermediate- and High-risk Prostate Cancer: Early Oncological Outcomes from a Randomized Phase 3 Trial. Eur Urol. 2021;79(5): 595-604.
- Hofman MS, Lawrentschuk N, Francis RJ, et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): a prospective, randomised, multicentre study. Lancet. 2020;395(10231):1208-1216.
- Hope TA, Eiber M, Armstrong WR, et al. Diagnostic Accuracy of 68Ga-PSMA-11 PET for Pelvic Nodal Metastasis Detection Prior to Radical Prostatectomy and Pelvic Lymph Node Dissection: A Multicenter Prospective Phase 3 Imaging Trial. JAMA Oncol. 2021;7(11):1635-42.
- Van Kalmthout LWM, van Melick HHE, Lavalaye J, et al. Prospective Validation of Gallium-68 Prostate Specific Membrane Antigen-Positron Emission Tomography/Computerized Tomography for Primary Staging of Prostate Cancer. J Urol. 2020;203(3):537-45.
- Jansen BHE, Bodar YJL, Zwezerijnen GJC, et al. Pelvic lymph-node staging with 18F-DCFPyL PET/CT prior to extended pelvic lymph-node dissection in primary prostate cancer - the SALT trial. Eur J Nucl Med Mol Imaging. 2021;48(2):509-20.
- Amiel T, Wurnschimmel C, Heck M, et al. Regional Lymph Node Metastasis on Prostate Specific Membrane Antigen Positron Emission Tomography Correlates with Decreased Biochemical Recurrence-Free and Therapy-Free Survival after Radical Prostatectomy: A Retrospective Single-Center Single-Arm Observational Study. J Urol. 2021;205(6): 1663-1670.
- Meijer D, van Leeuwen PJ, Donswijk ML, et al. Predicting early outcomes in patients with intermediate- and high-risk prostate cancer using prostate-specific membrane antigen positron emission tomography and magnetic resonance imaging. BJU Int. 2022;129(1): 54-62.
- Stabile A, Pellegrino A, Mazzone E, et al. Can Negative Prostate-specific Membrane Antigen Positron Emission Tomography/Computed Tomography Avoid the Need for Pelvic Lymph Node Dissection in Newly Diagnosed Prostate Cancer Patients? A Systematic Review and Meta-analysis with Backup Histology as Reference Standard. Eur Urol Oncol. 2022;5(1): 1-17.
- Touijer KA, Sjoberg DD, Benfante N, et al. Limited versus Extended Pelvic Lymph Node Dissection for Prostate Cancer: A Randomized Clinical Trial. Eur Urol Oncol. 2021;4(4): 532-539.


