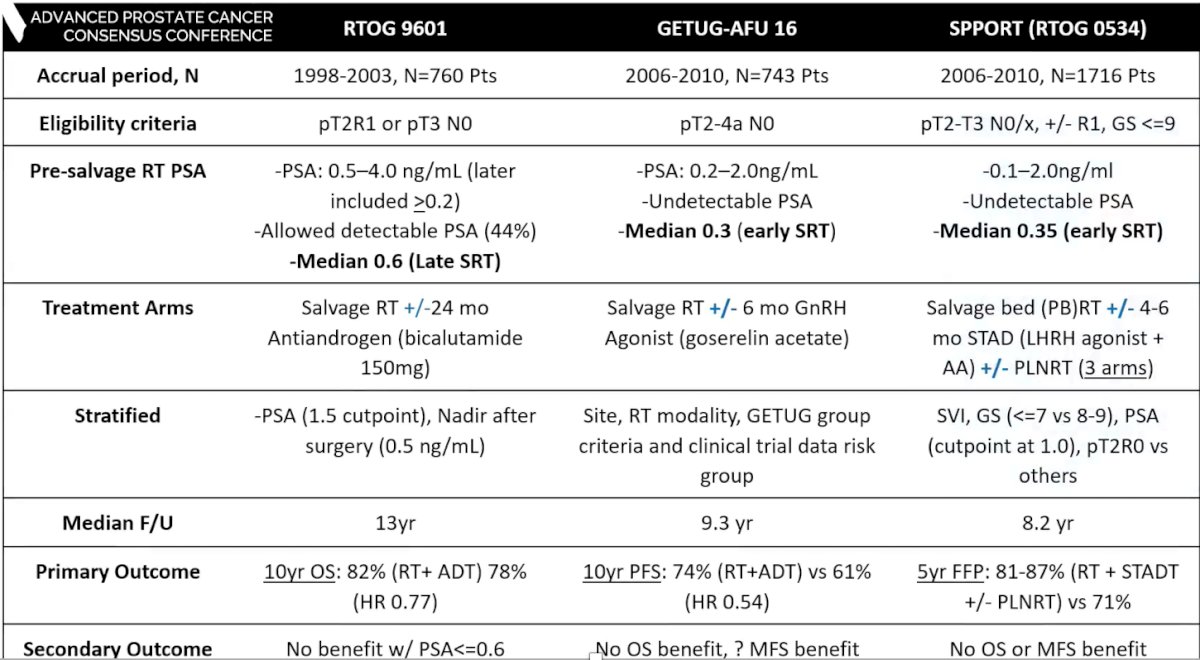
(UroToday.com) The 2022 Advanced Prostate Cancer Consensus Conference (APCCC) Hybrid Meeting included a session on biochemical recurrence and a presentation by Dr. Brandon Mahal discussing who needs systemic treatment with salvage radiotherapy. Dr. Mahal started his presentation by highlighting the RTOG 9601 trial,1 which was a phase III randomized clinical trial assessing bicalutamide monotherapy among salvage EBRT patients randomized to bicalutamide 150 mg PO daily vs placebo for 24 months. The overall survival rates at 12 years were 76.3% for patients taking bicalutamide compared to 71.3% taking placebo (p=0.04):

Prostate cancer survival rates at 12 years were 5.8% for bicalutamide compared to 13.4% for placebo (p<0.001). Furthermore, a subgroup analysis of this trial suggests that the ADT benefit is limited to PSA > 1.5 ng/mL:

Secondary analyses of RTOG 9601 show that pre-salvage radiotherapy PSA is both prognostic and predictive an ADT benefit:

Published in 2021 in JAMA Oncology,2 Dr. Feng and his team validated the Decipher genomic classifier in patients with recurrent prostate cancer using data from the RTOG 9601 randomized clinical trial. On multivariable analysis, the genomic classifier was independently associated with distant metastasis (HR 1.17, 95% CI 1.05-1.32; p = 0.006), prostate cancer specific mortality (HR 1.39, 95% CI, 1.20-1.63; p < 0.001), and overall survival (HR 1.17, 95% CI 1.06-1.29; p = 0.002) after adjusting for age, race/ethnicity, Gleason score, T stage, margin status, entry PSA, and treatment arm. Importantly, the absolute benefit from hormone therapy is smaller among the low Decipher risk group for the (i) entire cohort and (ii) early salvage radiotherapy (PSA < 0.7 ng/mL).
The next study discussed by Dr. Mahal was the GETUG/AFU-16 study assessing the role of early salvage radiotherapy.3 The objective of this trial was to establish the effect of adding short-term androgen suppression at the time of salvage radiotherapy on the biochemical outcome and overall survival in men with rising PSA following radical prostatectomy. This was an open-label, multicenter, phase 3, randomized controlled trial done at 43 French study centres. Patients were randomly assigned (1:1) to standard salvage radiotherapy (3D conformal radiotherapy or intensity modulated radiotherapy, of 66 Gy in 33 fractions 5 days a week for 7 weeks) or radiotherapy plus short-term androgen suppression using 10.8 mg goserelin by subcutaneous injection on the first day of irradiation and 3 months later. Overall, there were 743 patients randomly assigned, including 374 to radiotherapy alone and 369 to radiotherapy plus goserelin. Patients assigned to radiotherapy plus goserelin were significantly more likely than patients in the radiotherapy alone group to be free of biochemical progression or clinical progression at 5 years (80% [95% CI 75-84] vs 62% [57-67]; HR 0.50, 95% CI 0.38-0.66).
The third trial discussed by Dr. Mahal was the SPPORT (RTOG 0534) trial assessing the effect of early salvage radiotherapy (Lancet in press). This trial randomized 1,736 patients to either (i) salvage radiotherapy to the prostate bed, (ii) salvage radiotherapy to the prostate bed plus ADT, or (iii) salvage radiotherapy to the prostate bed plus ADT plus radiotherapy to the pelvic lymph nodes. The 5-year freedom from progression rate was 71% for salvage radiotherapy to the prostate bed, 81% for salvage radiotherapy to the prostate bed plus ADT, and 87% for salvage radiotherapy to the prostate bed plus ADT plus radiotherapy to the pelvic lymph nodes:

Based on only 108 patients with metastasis, there was minimal differences between the three arms with regard to metastasis free survival. It is likely that with continued follow-up, these results will likely to continue to favor salvage radiotherapy to the prostate bed plus ADT plus radiotherapy to the pelvic lymph nodes.
As follows is a summary of ADT with salvage radiotherapy trials:
Dr. Mahal notes that there are some “grey” zones that require clarification:
- What is the appropriate ADT duration (0, 4-6 months, 24 months) and for whom?
- We need to further clarify ADT benefit pre-salvage radiotherapy PSA, and ADT benefit by tumor biology/genomic classifier
- Should we use intensification of systemic therapy for high risk patients?
- How do we handle the incorporation of advanced imaging (PSMA PET/CT)?
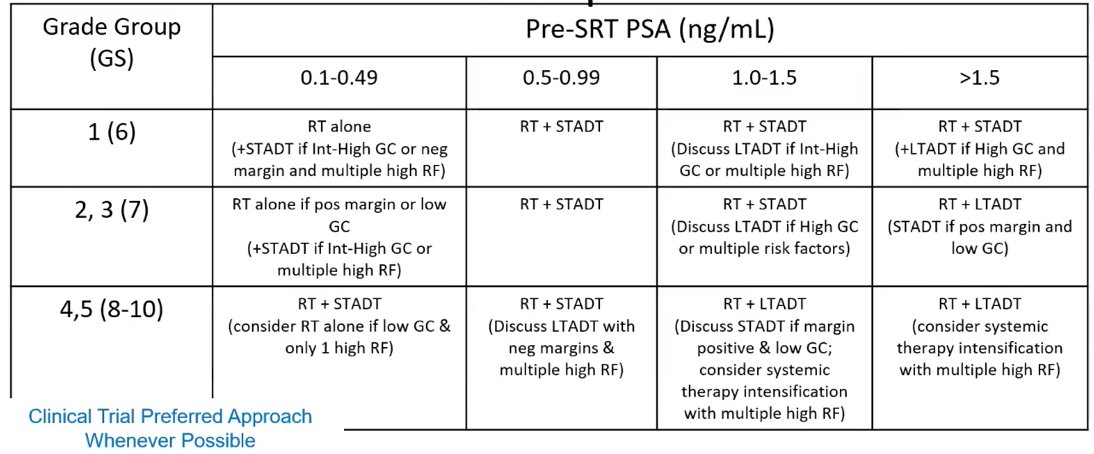
As follows is the risk adapted algorithm Dr. Mahal uses in his clinical practice:
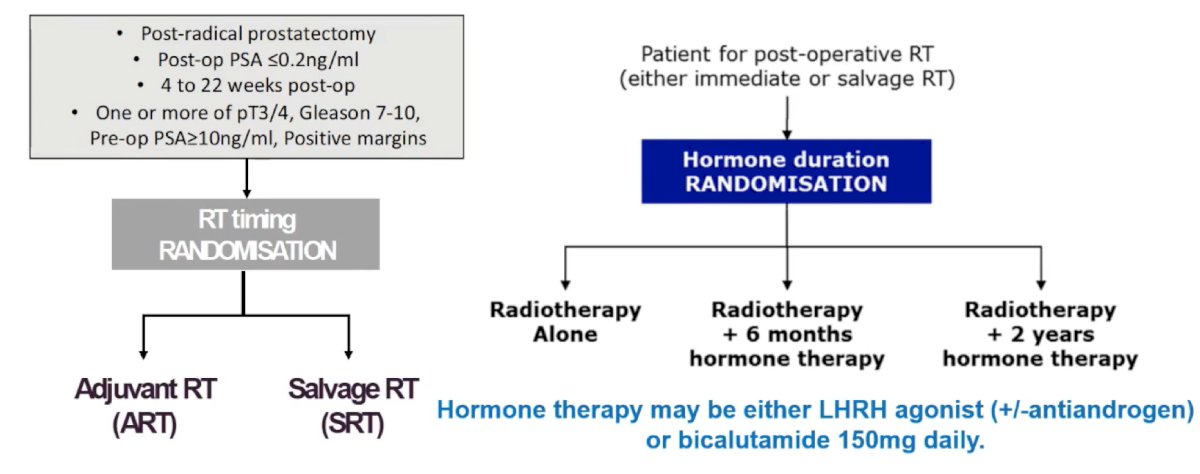
For the remainder of his presentation, Dr. Mahal discussed several ongoing clinical trials in this disease space. The RADICALS-HD trial is evaluating hormone therapy duration in ‘early’ post-op radiotherapy, with the following trial schematic:
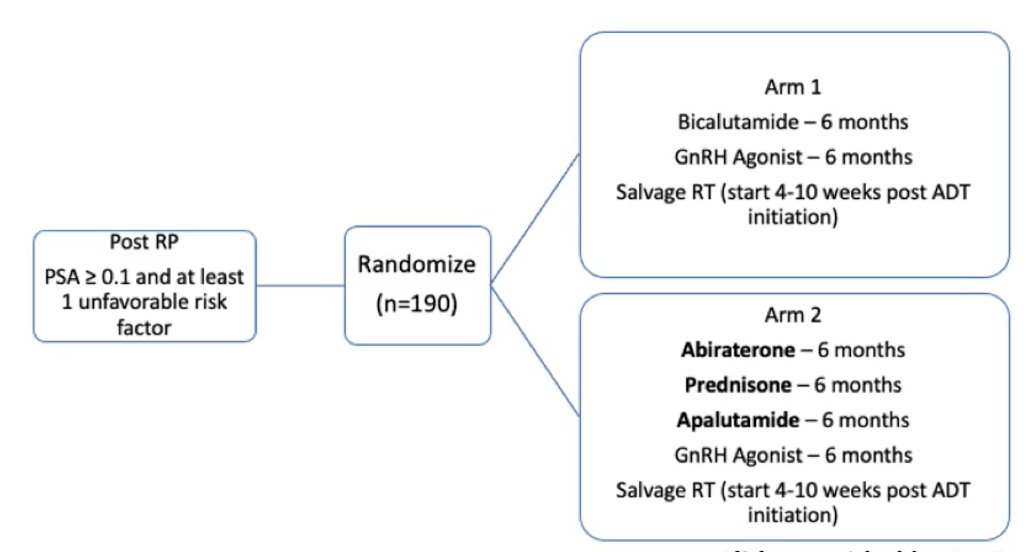
NRG GU-006 is a phase II biomarker enhanced anti-androgen trial for intermediate risk prostate cancer, utilizing biomarker stratified PAM 50. Men will be enrolled who have a PSA of 0.1-1.0 ng/mL (or persistent PSA after radical prostatectomy 0.04-0.2 ng/mL). Randomization will be to radiation versus radiation + apalutamide. The FORMULA-509 trial will assess enhanced androgen ablation and abiraterone + prednisone in intermediate/high risk patients:
The RTOG 3506 (STEEL) trial will assess standard versus enhanced ADT (for 24 months) in patients with high-risk prostate cancer:

Finally, NRG GU002 (RADD) will assess intensification with docetaxel for very high-risk prostate cancer patients:

Presented By: Brandon Mahal, MD, University of Miami School of Medicine, Sylvester Comprehensive Cancer Center, Miami, FL
Written By: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2022 Advanced Prostate Cancer Consensus Conference (APCCC) Annual Hybrid Meeting, Lugano, Switzerland, Thurs, Apr 28 – Sat, Apr 30, 2022.
References:
- Shipley WU, Seiferheld W, Lukka HR, et al. Radiation with or without Antiandrogen Therapy in Recurrent Prostate Cancer. N Engl J Med 2017;376(5):417-428.
- Feng FY, Huang HC, Spratt DE, et al. Validation of a 22-Gene Genomic Classifier in Patients with Recurrent Prostate Cancer: An Ancillary Study of the NRG/RTOG 9601 Randomized Clinical Trial. JAMA Oncol. 2021 Apr 1;7(4):544-552.
- Carrie C, Hasbini A, de Laroche G, et al. Salvage radiotherapy with or without short-term hormone therapy for rising prostate-specific antigen concentration after radical prostatectomy (GETUG-AFU 16): A randomized, multicentre, open-label phase 3 trial. Lancet Oncol 2016;17(6):747-756.


