(UroToday.com) In the session of the 2022 Advanced Prostate Cancer Consensus Conference focusing on the treatment of patients with oligometastatic and oligoprogressive prostate cancer, Dr. Beer discussed the question of how to follow patients in whom PSMA-PET imaging has been used for baseline staging or diagnostic imaging. In this context, he noted that there are a number of potential use cases, including repeat imaging in the biochemical recurrence setting with rising PSA, for follow-up of metastasis-directed therapy in oligometastatic disease, for follow-up of systemic therapy (including novel hormonal agents, cytotoxic or immunotherapies, and PSMA-directed radioligand or adoptive cellular therapies). Currently, approved indications for PSMA-PET imaging are more of a “snap shot in time” across a variety of clinical scenarios, rather than these longitudinal case examples. As a result, the literature to support this approach is somewhat sparse.
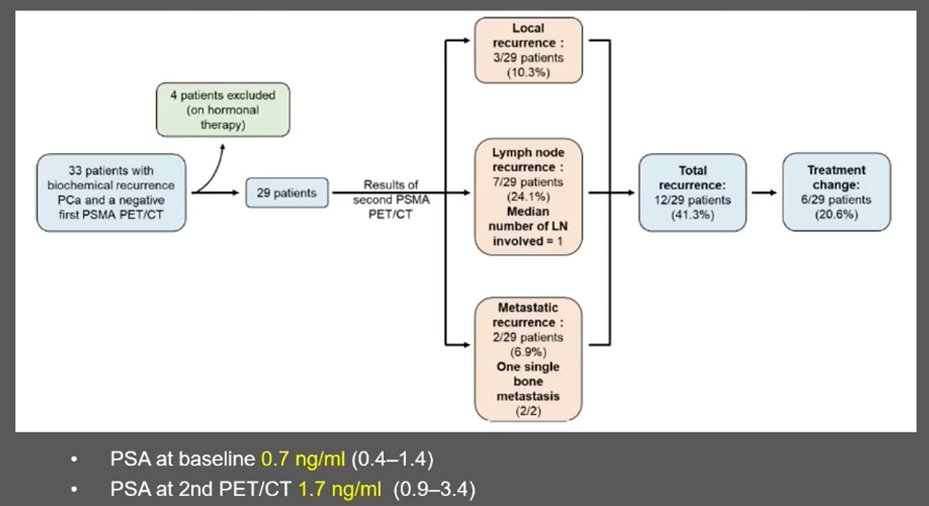
However, Dr. Beer launched into what is known about the role of serial PSMA-PET in patients with biochemical recurrence. Among patients with biochemically recurrent disease (median PSA 0.7 ng/mL), 29 men with negative PSMA-PET underwent a second PSMA-PET, typically about 6 months following the first. At this time, the median PSA had risen to 1.7 ng/mL and radiographically apparent recurrent disease was identified in 12 of the 29 patients (41%) resulting in a treatment change in 6 (21%).
He noted that in the case of the follow-up of PSMA image-guided oligometastatic metastasis directed therapy, there is little guidance. Certainly, among the limited literature available, the vast majority of patients with rising PSA following metastasis-directed therapy had out-of-field relapses at the time of repeat PSMA-PET. Thus, this raises the spectre of repeated metastasis-directed therapy. In one small study, 7 of 32 patients treated in this manner were biochemically free of disease after this second round of lesion-directed therapy.
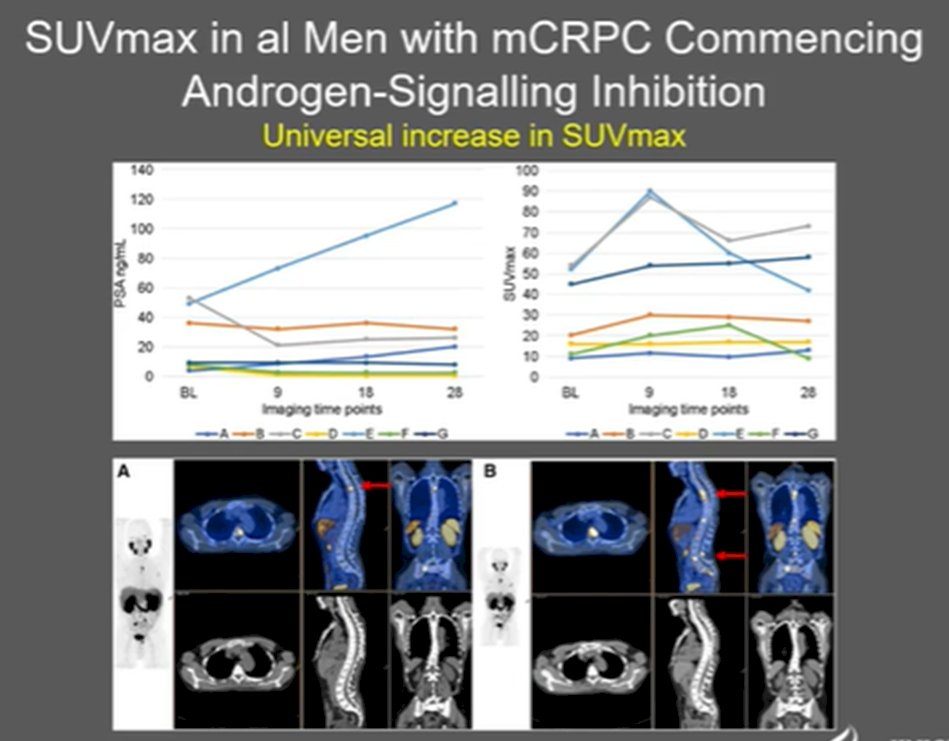
He noted, however, that the serial use of PSMA-PET imaging for assessing response to systemic therapies may be complicated by changes in androgen signalling that may confound PSMA imaging. In particular, use of ADT may increase PSMA imaging signal in the short-term as anti-androgen administration can increase PSMA mRNA transcription. When assessed clinically with serial 68Ga-PSMA-11 at baseline and then 9-, 18-, and 28-days following the initiation of systemic therapy for advanced prostate cancer (either ADT for hormone sensitive disease or abiraterone/enzalutamide for metastatic castration resistant disease), there were notable changes. Among the men starting on ADT, there was considerable heterogeneity in PSMA SUVmax, despite consistent decreases in PSA.

In contrast, among men starting abiraterone or enzalutamide, there were relatively consistent increases in SUVmax.
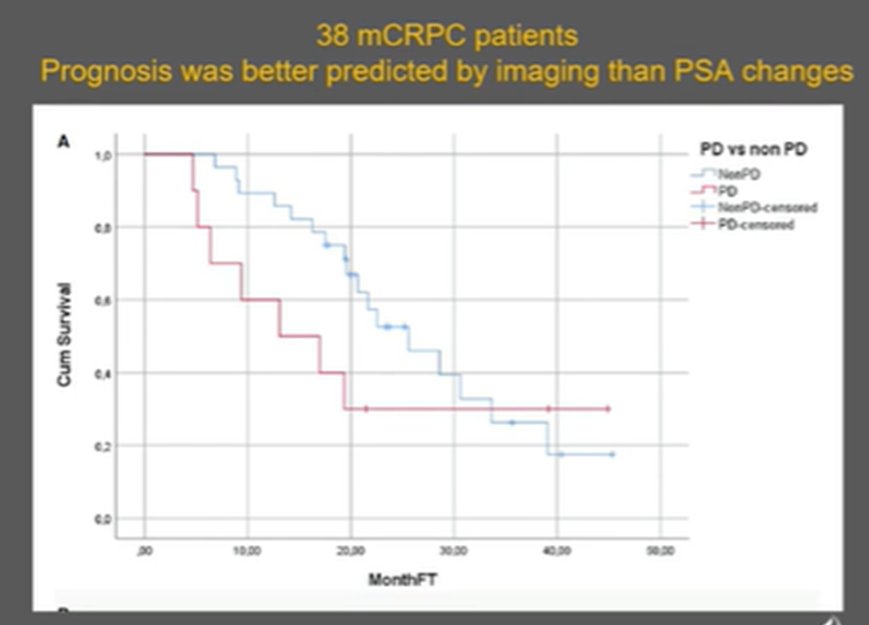
Similar work from Dr Agarwal has shown that, lesion-by-lesion, there may be interlesional heterogeneity in terms of changes in signal within the same patient. However, after prolonged androgen deprivation therapy (median 155 days), both the number and overall volume of PSMA-expressing tumor lesions is expected to decrease dramatically, with a 80% decrease in tumor volume in one recent study. For those patients treated with taxane-based chemotherapy, the PSMA response correlated well with PSA response and was also correlated with overall survival. Among 29 men with mCRPC in whom PSMA-PET was performed at baseline and following the last cycle of taxane, the median overall survival was 22 months in those with a PSMA-response and 12 months in non-responders by PSMA. A similar effect can be observed for patients being treated with PSMA-directed therapies in which case imaging changes were most predicting of overall prognosis.
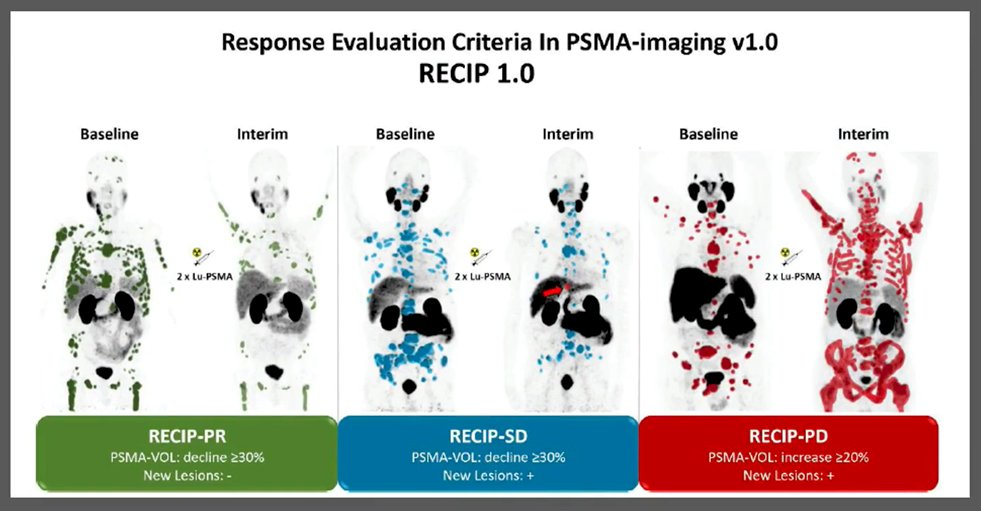
Moving forward, Dr. Beer emphasized the value of assessments of overall tumor burden, rather than simply counting sites. To do this, software will be essential to quantify the volume of PSMA-expressing disease. This approach offers an opportunity for very early evaluation nof treatment response, as highlighted in the recent publication outlining RECIP 1.0, a framework for evaluation treatment response using PSMA-PET/CT in patients with metastatic castration resistant prostate cancer. Premised on a RESIST-type approach, this framework uses the volume of PSMA-expressing disease to categorize patients as having progressive disease (median OS 8.3 months), stable disease (median OS 13.1 months), partial response (median OS 21.7 months), or complete response (which was not observed in this study of 124 men). Notably, a large portion of patients with progressive disease by RECIP 1.0 did not have evidence of progression on conventional CT imaging.
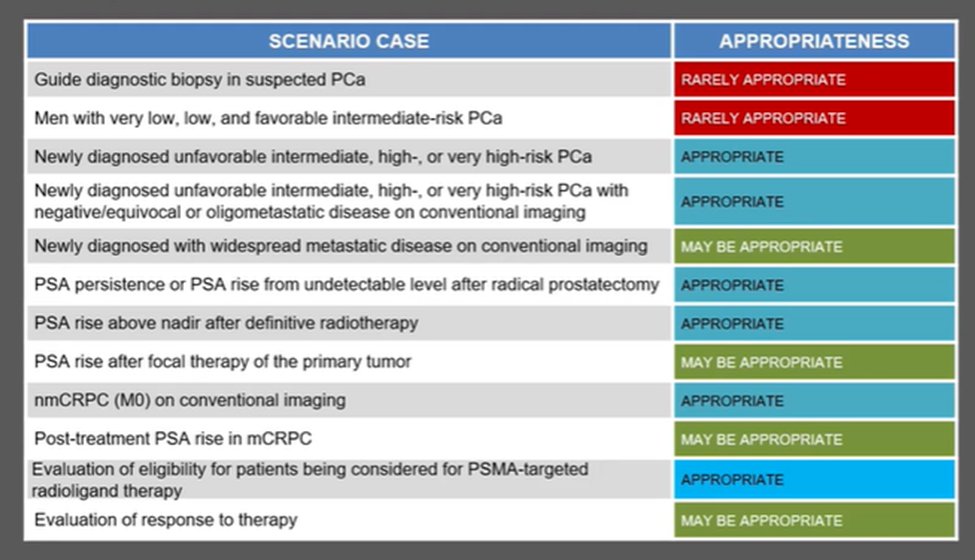
While this approach offers great promise, he noted that by and large, we are relying on small studies. However, the SNMMI has assessed a variety of clinical scenarios to help guide when use of PSMA-PET is appropriate. The evaluation of response to therapy, currently, falls into the “may be appropriate” category.
Moving forward, Dr. Beer emphasized that there are many outstanding questions regarding the use of PSMA-PET/CT response criteria, some of which have been addressed in a recent consensus statement. However, he felt that the use of this approach is nearly inevitable. Importantly, though, the information from this repeat imaging may not convey what we think it does as changes in PSMA-imaging results may relate to changes in tumor volume or changes in PSMA expression. Further, there may be treatment modality specific patterns of change that need to be better understood. He suggested that imaging should be assessed in the same framework as we consider biomarkers, focusing on identifying data to support the relationship between changes in the biomarker and clinical outcomes of interest.
Presented by: Tomasz M. Beer, MD, FACP, Professor of Medicine, Division of Hematology/Medical Oncology, School of Medicine, Grover C. Bagby Chair of Prostate Cancer Research, OHSU Knight Cancer Institute Deputy Director, OHSU Knight Cancer Institute, School of Medicine, Chief Medical Officer, CEDAR, OHSU Knight Cancer Institute, School of Medicine, Cancer Biology Graduate Program, School of Medicine

