(UroToday.com) The 2022 Advanced Prostate Cancer Consensus Conference (APCCC) Hybrid Meeting included a session on the management of metastatic hormone sensitive prostate cancer (mHSPC) and a presentation by Dr. Andrew Armstrong discussing the uptake of new treatment options for mHSPC in real life, including education, access, use, and diversity. Dr. Armstrong notes that men who present with metastatic disease (5-10% of patients in the US make up mHSPC) unfortunately face a shorter natural history. Earlier use of potent androgen receptor therapy is changing our algorithm for subsequent therapy, balancing the integration of chemo-hormonal therapy with ADT or novel androgen receptor therapies. The tumor volume and activity vs time curve for the landscape of advanced prostate cancer is as follows:

In 2022, there is an extensive list of mHSPC therapies available with proven survival benefits:
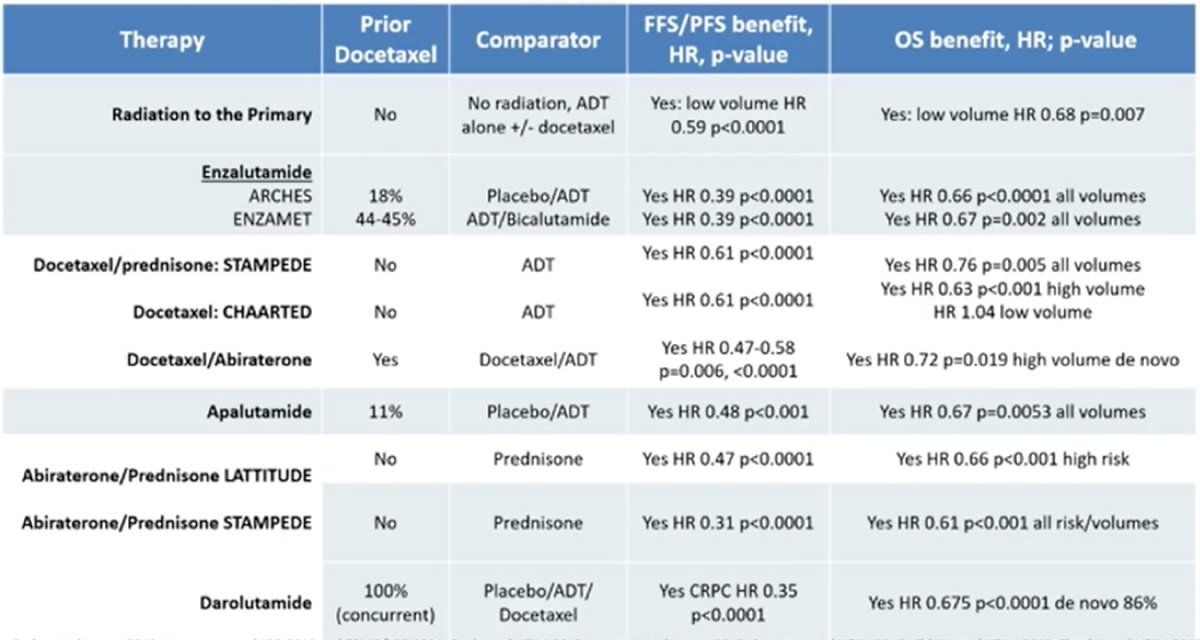
With so many options available, it can be difficult to decide when/how to sequence therapy. Dr. Armstrong notes that the following algorithm can assist with triaging lines of therapy, stratified by nmHSPC (localized high/very high NCCN risk) vs mHSPC low volume/risk (or N1M0) vs mHSPC high volume/risk:
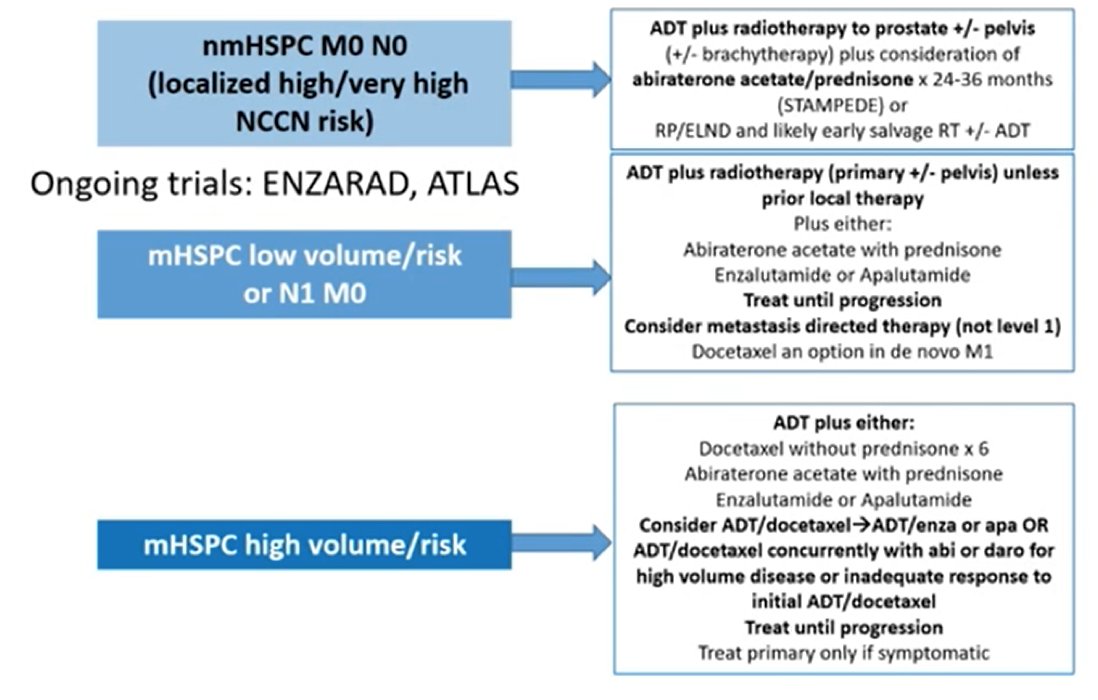
Similar stratifications have also been provided by the NCCN and ASCO treatment guidelines. Given that our trials should be providing information on efficacy and safety for the patients that will subsequently benefit from new treatment on a larger scale, it is important for us to ask whether our clinical trials of men with mHSPC are representative of that population. As highlighted in the following table, particularly for African Ancestry (low enrolment in key mHSPC clinical trials), the answer is most likely no, we are not representing our populations adequately:

To further highlight this lack of representation of minorities in US prostate cancer trials, a recent study suggested over-representation of white men (RQ 1.15) and an under-representation of Black men (RQ 0.63).1 This disparity was particularly notable in industry trials (RQ 1.18 for white men vs 0.34 for Black men). Presently, the burden of lethal prostate cancer is situated in the Caribbean, sub-Saharan Africa, and South America:

Several recent studies using data from both the VA and insurance claims databases have suggested that treatment intensification is poor for mHSPC patients, with nearly 2/3 of patients still receiving ADT alone, consistent across all races. Even with the most recent data (2019-2020), 45% of men with mHSPC were not receiving treatment intensification:

Recent work from Kensler et al.2 has assessed the impact of screening trends on mHSPC. Using the Behavioral Risk Factor Surveillance System (2012-2018), this study assessed the proportion of 40-74 year old men who self-reported receiving a routine PSA test. In 2012, PSA testing frequencies were 32.3% (95% CI 31.7% to 32.8%) among non-Hispanic White men, 30.3% (95% CI 28.3% to 32.3%) among Black men, 21.8% (95% CI 19.9% to 23.7%) among Hispanic men, and 17.7% (95% CI 14.1% to 21.3%) among Asian and Pacific Islander men. The absolute screening frequency declined by 9.5% from 2012 to 2018 with a larger decline among Black (11.6%) versus non-Hispanic white men (9.3%). Coupled with decreased screening, metastatic disease incidence is rising across all racial and ethnic groups. Furthermore, COVID-19 has exacerbated this screening/early detection deficit and disparity.
Looking at UK national data, Dr. Armstrong notes that 13% of 45,000 men with prostate cancer present with M1 disease, with a 54% reduction in the number of men that presented with newly diagnosed prostate cancer during the peak of the COVID-19 pandemic (April-June 2020). This was followed by an increase thereafter, but without return to the pre-pandemic levels. Additionally, M1 disease at diagnosis has increased to 21% since April 2020, coupled with a 45-48% reduction in radial prostatectomies and radiotherapy volumes in April-June 2020, and a 20-25% reduction in the subsequent 6 months. There was also a rapid decrease in the use of docetaxel in April 2020 in the mHSPC setting, with a rapid and marked increased use of enzalutamide.
Dr. Armstrong then discussed the Duke Prostate Cancer Screening Algorithm to potentially reduce the mHSPC disease burden.3 During pre- and post-implementation periods, 49,053 and 49.980 men, respectively, were seen across 26 clinics (20.6% African American). The proportion of men who met screening algorithm criteria increased from 49% to 68% after implementation (p < 0.001), with similar effects in all race/ancestry groups. As follows is the risk-stratified cancer screening algorithm:

Even among mCRPC trials there is a lack of inclusion of black men in clinical trials, with enrollment estimates ranging from 2.0%-5.3% with an estimated expected Black men enrollment of 15.8%. Dr. Armstrong emphasized that we need to do better, and a mandate to allocate enrollment in US (and global) trials to disproportionately impacted groups in prostate cancer trials should be considered.
Given the disparities in Black men enrolling in prostate cancer clinical trials, a recent trial lead by Dr. Armstrong’s team at Duke assessed abiraterone acetate plus prednisone to explore outcomes by race (50 White vs 50 Black) among patients with mCRPC.4 The median rPFS for Black and White patients was 16.6 and 16.8 months, respectively, their times to PSA progression (TTP) were 16.6 and 11.5 months, respectively, and their OS was 35.9 and 35.7 months, respectively. Estimated rates of PSA decline by ≥50% in Black and White patients were 74% and 66%, respectively, and PSA declines to <0.2 ng/mL were 26% and 10%, respectively. Thus, despite higher comorbidity rates, Black patients demonstrated rPFS and OS similar to those of White patients and trended toward greater TTP and PSA declines. However, inclusion matters, as rates of grade 3 and 4 hypertension, hypokalemia, and hyperglycemia were higher in Black men:

Dr. Armstrong then listed several gray areas for APCCC discussion as follows:
- The role of novel imaging (PSMA or Fluciclovine PET/CT) to detect occult metastases
- How and when should we treat the primary (surgery or radiation) in the face of M1 disease based on standard versus PET imaging?
- Should we treat oligometastatic sites and how should we integrate metastasis directed therapy with the best systemic therapy?
- Can we stop systemic therapy if all of the disease has been treated?
- When to choose a potent androgen receptor inhibitor versus docetaxel plus ADT and which one? What is the optimal therapy in resource constrained regions, such as orchiectomy plus abiraterone 250 mg/prednisone with food?
- How to integrate docetaxel with concurrent or sequential androgen receptor inhibition in mHSPC? Real world effectiveness studies are needed, particularly assessing the financial impact of care and improving inclusion of disproportionately impacted minority populations
- How to follow patients and reduce the impact on quality of life while optimizing survival?
- Impact of initial choice on subsequent therapy due to cross-resistance
Dr. Armstrong concluded his presentation emphasizing that there are unmet needs to potential steps to improve inclusion and treatments globally:
- Cost of oral agents: (i) development of registries, expanded access datasets to improve data on efficacy/safety in under-represented regions, (ii) lower cost options including ¼ dose abiraterone with a low-fat diet (bioequivalent), and (iii) intermittent approaches in low volume patients achieving remission (ALLIANCE concept trial A032101)
- Reductions in long-term systemic therapy needs: (i) improved access to curative intent therapies, screening/early detection in diverse populations to avoid mHSPC altogether, and (ii) improved availability of more intensive hormonal therapies for shorter courses (6 months – 2 years) in curative intent settings to reduce the risk of metastasis and death
- Required representation of disproportionately impacted populations in clinical trials based on recent FDA guidance
Presented By: Andrew Armstrong, MD, Duke Cancer Institute, Duke University, Durham, NC
Written By: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2022 Advanced Prostate Cancer Consensus Conference (APCCC) Annual Hybrid Meeting, Lugano, Switzerland, Thurs, Apr 28 – Sat, Apr 30, 2022.
References:
- Owens-Walton J, Williams C, Rompre-Brodeur A, et al. Minority enrollment in phase II and III Clinical Trials in Urologic Oncology. J Clin Oncol 2022 Feb 23 [Epub ahead of print].
- Kensler KH, Pernar CH, Mahal BA, et al. Racial and ethic variation in PSA testing and prostate cancer incidence following the 2012 USPSTF recommendation. JNCI 2021;113(6):719-726.
- Shah A, Polascik TJ, George DJ, et al. Implementation and impact of a risk-stratified prostate cancer screening algorithm as a clinical decision support tool in a primary care network. J Gen Intern Med. 2021 Jan;36(1):92-99.
- George DJ, Halabi S, Heath EI, et al. A prospective trial of abiraterone acetate plus prednisone in Black and White men with metastatic castrate-resistant prostate cancer. Cancer 2021 Aug 15;127(16):2954-2965.


