(UroToday.com) In this session, Dr. Bertrand Tombal and Dr. Nick James summarized the panel responses to the consensus questions. This was followed by a discussion on the management of metastatic hormone sensitive prostate cancer (mHSPC) with input from the discussants and the panel.
Question #12 focused on the preferred systemic therapy in addition to ADT for men with synchronous high-volume mHSPC. 49% answered an AR pathway inhibitor (Abi/Apa/Enza) as sole additional therapy, 40% answered docetaxel plus as AR pathway inhibitor, and 11% answered docetaxel as the sole additional therapy. Dr, Karim Fizazi was asked to comment on why he thinks less than 50% of panel members did not choose triplet therapy. Dr. Fizazi acknowledged that the PEACE-1 data is brand new and it can take time for people to analyze new data and decide if its practice changing. He believes that it is for this patient population.
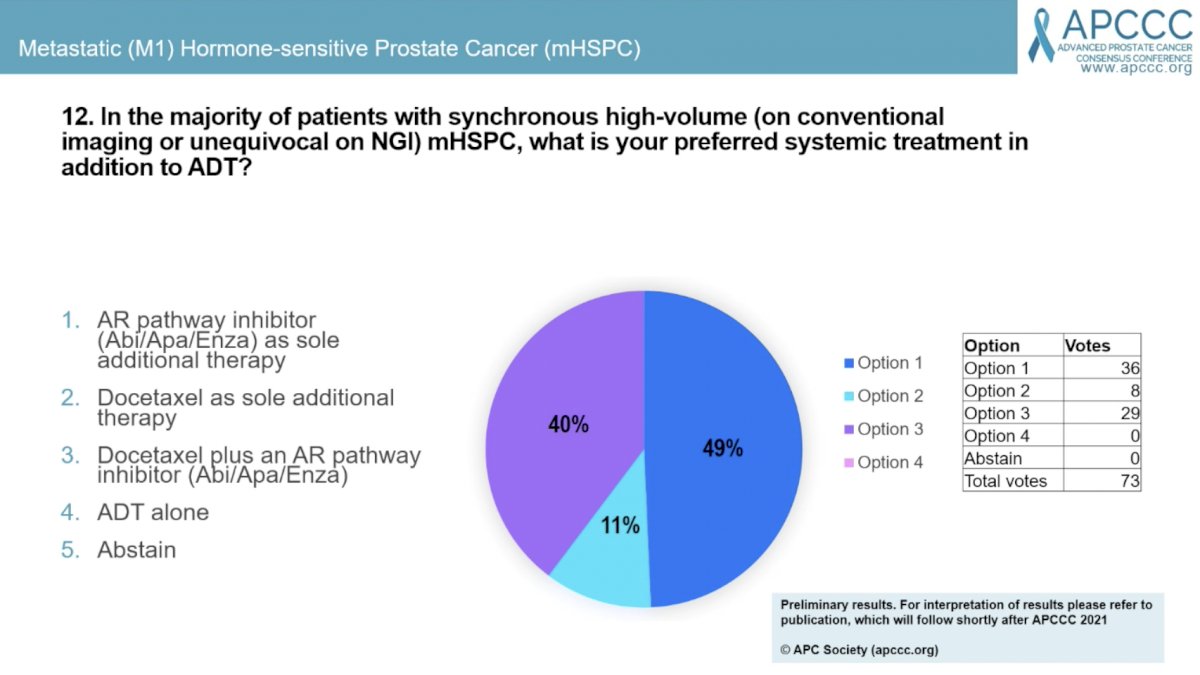
Question #13 asked about preferred systemic therapy in addition to ADT for men with metachronous high-volume mHSPC. A larger majority (71%) elected for an AR pathway inhibitor alone with 21% opting for docetaxel plus and AR pathway inhibitor and 7% for docetaxel as sole additional therapy. Dr. Fizazi acknowledged that PEACE-1 only included men with de novo mHSPC, so it’s not completely clear how to treat men with high-volume metachronous mHSPC. Panel members commented that this is a relatively uncommon clinical scenario.
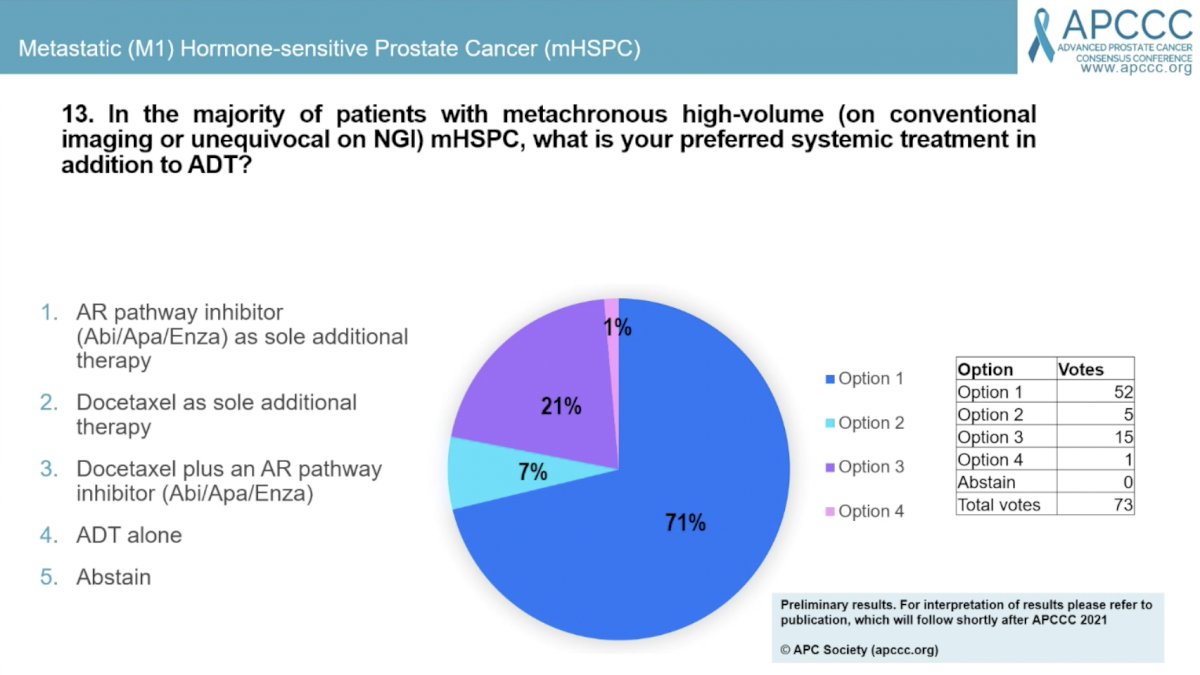
Question #29 asked “in which patients with synchronous mHSPC that are chemotherapy fit do you recommend the triplet therapy of ADT plus docetaxel plus abiraterone (in addition to ADT)? 55% answered “only in high-volume,” 41% answered “I usually do not recommend this combination,” and 4% answered “in the majority of patients independent of disease volume.” Dr. Fizazi commented on the strong radiographic progression-free survival (rPFS) from men with high- and low-volume disease. While the overall survival (OS) data only showed a statistically significant benefit in those with high-volume disease, the low-volume OS data is immature and further follow-up is needed to determine the benefit in this patient population.
In response to a question about how rPFS was defined, Dr. Fizazi commented that rPFS included only radiographic progression or death, and not PSA or clinical symptoms. Men were followed by PSA and imaging was started as soon as men had PSA progression or symptomatic progression. It was recommended that men undergo imaging at least every 6 months, however, clinicians could change therapy as soon as they wanted and for any reason.
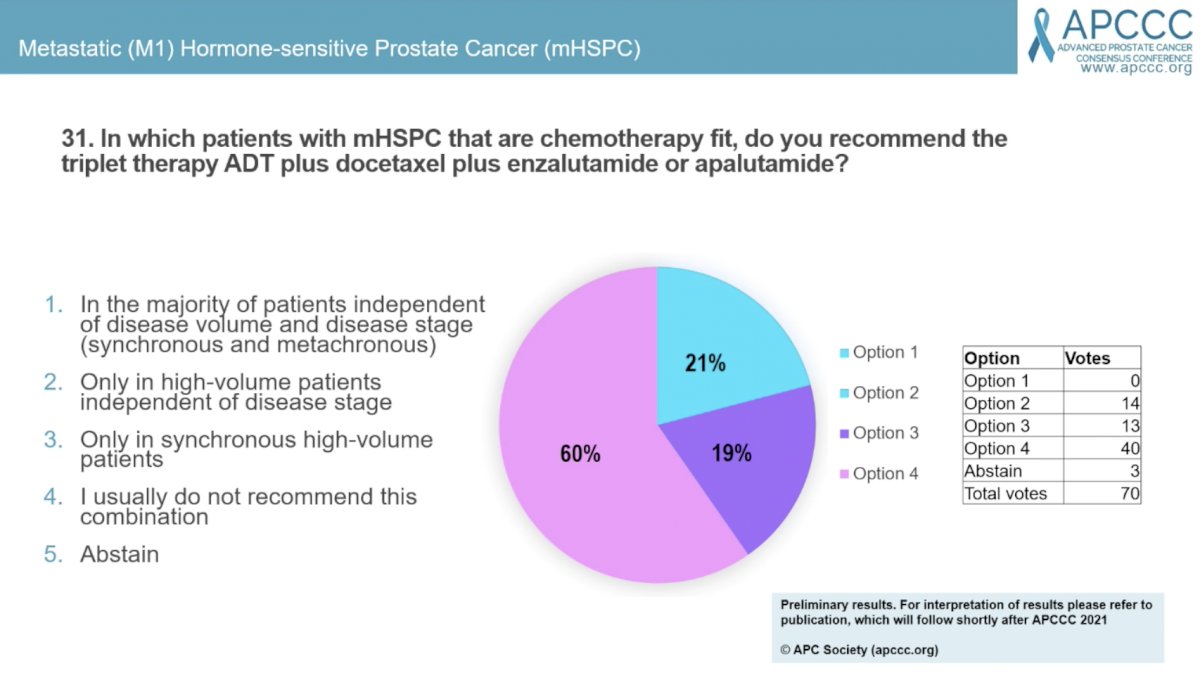
Question #31 asked for which patients with mHSPC that are chemotherapy fit do the panel members recommend triplet therapy of ADT plus docetaxel plus enzalutamide or apalutamide? The majority (60%) answered “I usually do not recommend this combination,” while 21% answered “only in high-volume patients independent of disease stage,” and 19% “only in synchronous high-volume patients.”
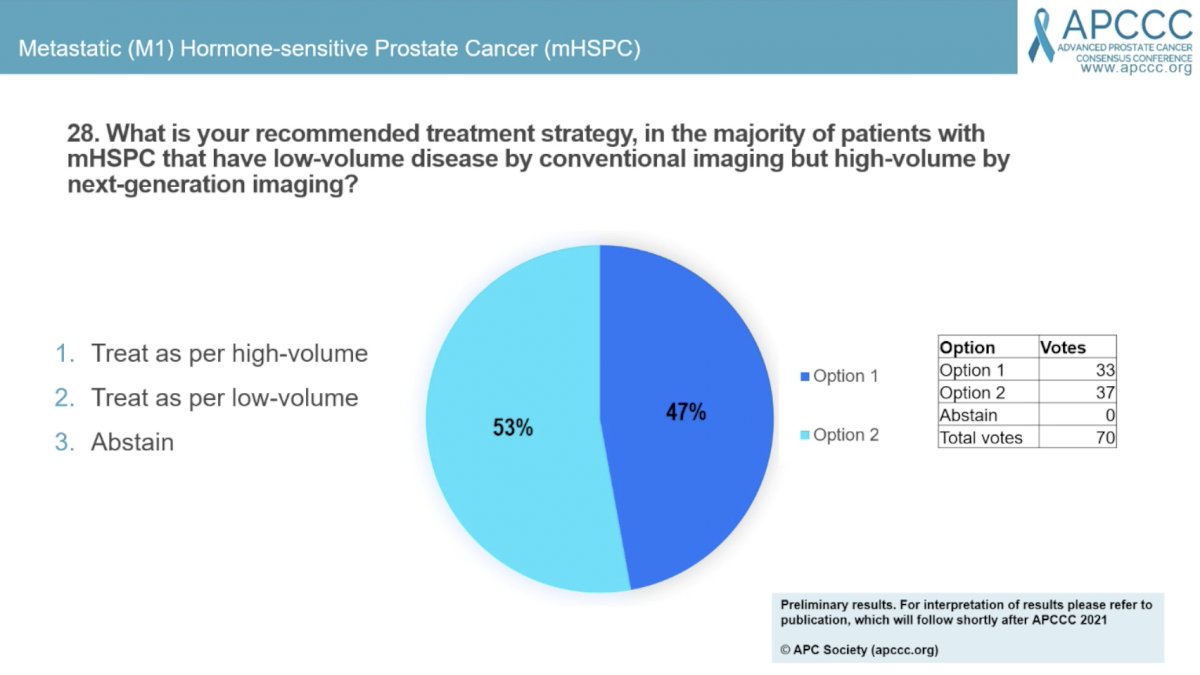
Question #28 asked “What is your recommended treatment strategy in the majority of patients with mHSPC that have low-volume disease by conventional imaging, but high-volume by next-generation imaging?” A slight majority (53%) answered “treat as low-volume” and the remaining 47% would “treat as high-volume.” Dr. Chris Parker expressed concern that so many people would treat as high-volume and may therefore offer RT to the primary prostate. He stressed that the survival benefit of RT in low-volume mHSPC is substantial, especially when considering it is cheap and safe. He cautioned about withholding this life-prolonging therapy when we don’t yet have a definition of low- versus high-volume with advanced imaging yet.
The next question (#15) asked what the preferred treatment in addition to ADT is for the majority of patients without symptoms from the primary tumor with synchronous low-volume (on conventional imaging) mHSPC? The majority (77%) would treat with radical local treatment to the primary tumor plus additional systemic therapy with or without metastasis-directed therapy. This was the only question in this session that reached consensus.

Question #6 asked about the panel for the cut-off of number of bone metastases based on conventional imaging for recommending local treatment to the primary tumor in mHSPC. The majority (64%) answered 3 or less, 29% answered 5 or less, and 6% said there was no upper limit of bone metastases. Dr. Nick James commented that the pre-specified volume criteria for the STAMPEDE trial that addressed this question was 3 or less based on the CHAARTED criteria as this was the most widely accepted volume criteria at the time. The investigators hypothesized that patients with lower volume disease would more benefit more and this was borne out in the results. A subsequent exploratory analysis of benefit by number of bone metastases saw that the OS benefit is lost around 5 bone metastases. Dr. Fred Saad posed the question of whether we should be increasing the number from 3 to 5 or more now that we are adding intensified systemic therapy. Dr. Chris Parker commented that his approach is to err on the side of giving RT if there’s doubt as it is relative safe and there is a strong suggestion of OS benefit in men with 4 or 5 bone metastases.

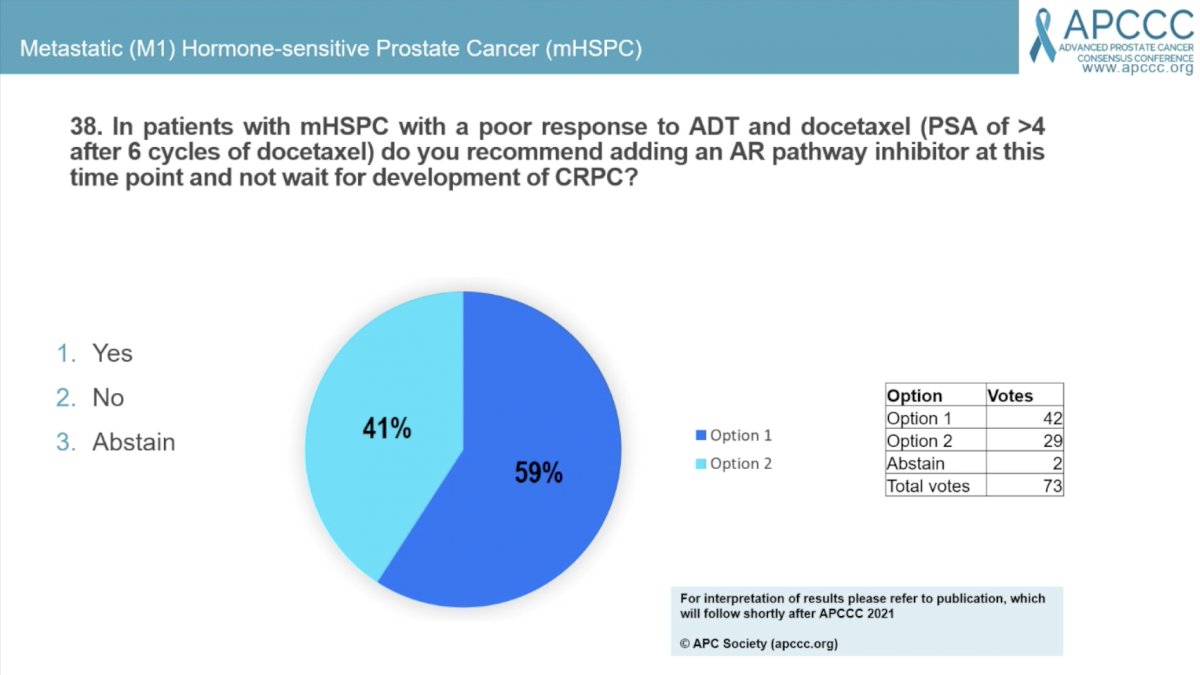
Question #38 asked “In patients with mHSPC with a poor response to ADT and docetaxel (PSA of >4 after 6 cycles) do you recommend adding an AR pathway inhibitor at this time point and not wait for development of CRPC?” 59% answered “yes” and 41% answered no.
Question #39 asked whether panel members discuss the possibility of stopping all systemic therapy in patients with mHSPC who durable deep remission to systemic treatment with PSA undetectable (e.g., <0.2 at 2-3 years). 61% of panel members answered yes and 39% answered no.

Presented by: Nick James, MBBS, PhD, Professor of Clinical Oncology in the Institute of Cancer and Genomic Sciences at the University of Birmingham and Bertrand Tombal, MD, PhD, Professor of Physiology and Chair of the Division of Urology at the Université Catholique de Louvain
Written by: Jacob Berchuck, MD, Genitourinary Medical Oncologist, Dana-Farber Cancer Institute (Twitter: @jberchuck) during the 2021 Advanced Prostate Cancer Consensus Conference, Saturday, October 9, 2021.


