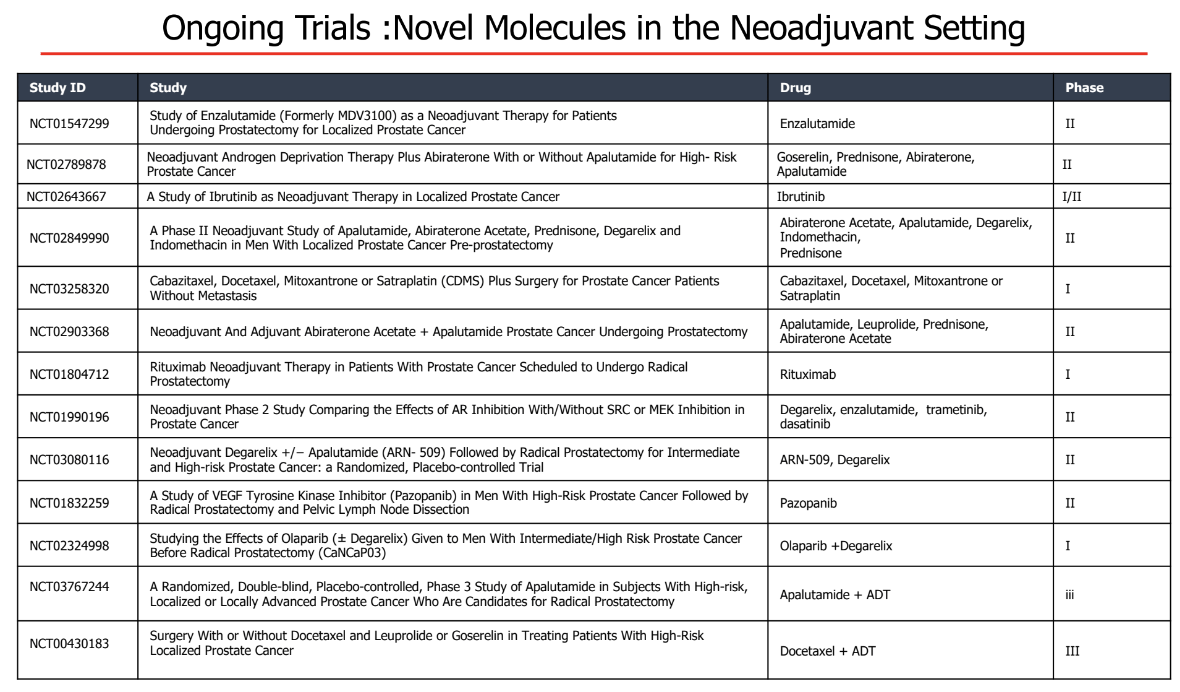
Table 1 – Ongoing trials assessing novel molecules in the neoadjuvant setting:
Another option to pursue is the use of various genomic tests such as the DECIPHER test to select ideal candidates for postoperative radiotherapy. The DECIPHER test is an independent predictor of metastases after adjusting for other variables.1 Aside from pathological stage T3b/T4, Gleason scores above 8, and lymph node invasion, a Decipher score of above 0.6 has been shown to predict recurrence.2 There are also several ongoing trials assessing genomic-driven adjuvant therapies, such as the PRO-IMPACT trial evaluating the clinical utility of Decipher for the administration of postoperative therapies (NCT02080689).
There is also an ADT response signature (ADT-RS). It has been created from gene expression patterns that may serve as an early marker of androgen resistance. Patients with a low ADT-RS had similar 10-year metastasis rate in those treated with ADT compared to those not treated with ADT. In contrast, those with a high ADT-RS, treatment with ADT conferred significantly lower 10-year metastasis rate than those not treated with ADT (9.4% vs. 29.2%, p=0.02).3
The second issue discussed was image-guided surgery. Despite its increasing use and enhanced accuracy, the PET-PSMA has a sensitivity of 24.4% per node, and 38.2% per patient, which is quite low.4 Therefore, PET-PSMA will not be able to replace an anatomically defined extended pelvic lymph node dissection. Incorporation of the PET-PSMA imaging during surgery, creating a radio-guided surgery, will enhance our accuracy and allow us to remove all involved lymph nodes (Figure 1).
Figure 1- Radio-guided surgery:

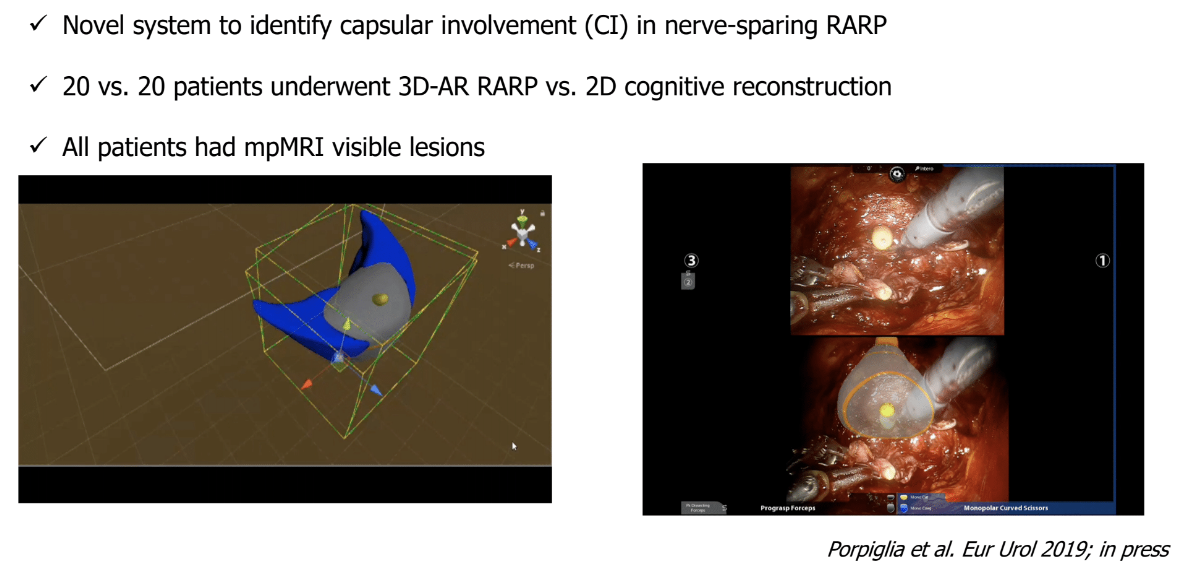
The third issue discussed by Dr. Briganti was the use of novel tools to improve local control. These include three-dimensional elastic augmented-reality robotic radical prostatectomy using hyper-accuracy three-dimensional reconstruction technology. This is a novel system used to identify capsular involvement when performing nerve-sparing surgery. This allows incorporating the MRI images into the robotic console during surgery and enhancing accuracy during surgery (Figure 2).
Figure 2- Three-dimensional elastic augmented-reality robotic radical prostatectomy using hyper-accuracy three-dimensional reconstruction technology.
The fourth and last point was centralization of care. A large population-based study has shown that the overall survival of radical prostatectomy surgeries improved in direct correlation to the facility volume.5 Therefore, surgeries of high-risk patients should be performed strictly in high-volume centers.
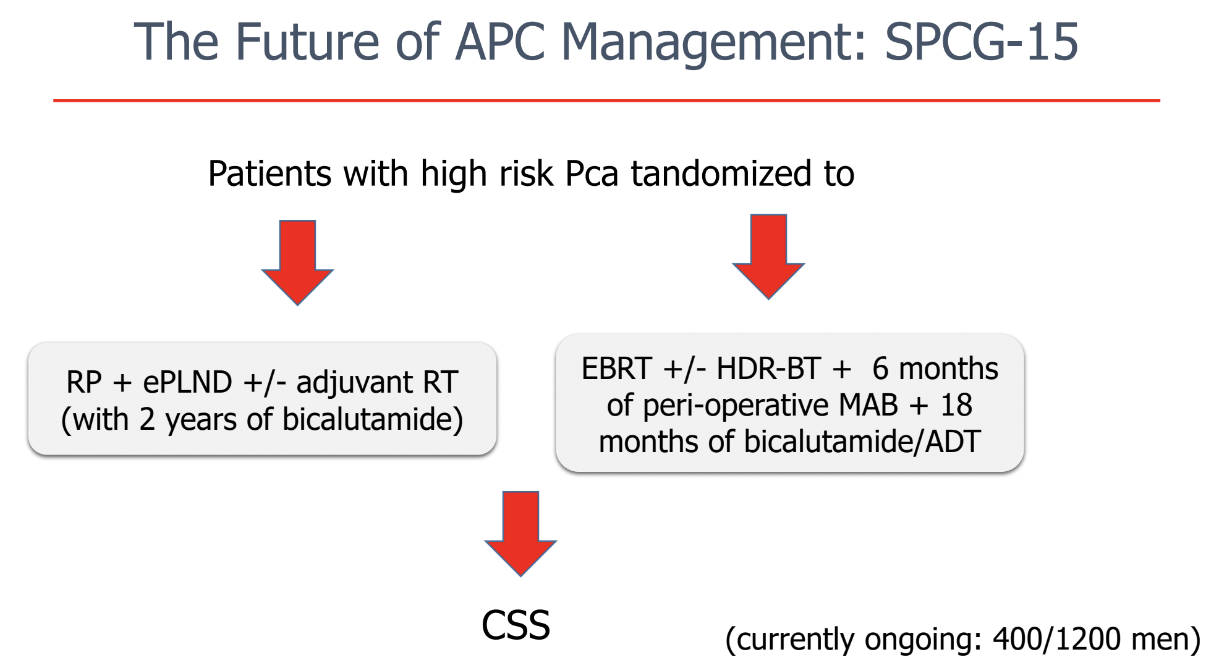
Dr. Briganti concluded his talk mentioning the currently ongoing SPCG-15 trial which will randomize patients with high-risk prostate cancer to surgery + extended pelvic lymph node dissection + adjuvant radiotherapy with 2 years of bicalutamide, and to radiotherapy + ADT for 18 months (Figure 3).
Figure 3 – SPCG-15 trial:
Presented by: Alberto Briganti, MD, Professor of Urology, Università Vita-Salute San Raffaele, Deputy Director, Urological Research Institute (URI) of IRCCS Ospedale Università Vita-Salute San Raffaele, Milan, Italy
Written by: Hanan Goldberg, MD, Urology Department, SUNY Upstate Medical University, Syracuse, New York, USA @GoldbergHanan at the 2019 Advanced Prostate Cancer Consensus Conference (APCCC) #APCCC19, Aug 29 - 31, 2019 in Basel, Switzerland
References:
- Spratt DE, Yousefi K, Deheshi S, et al. Individual Patient-Level Meta-Analysis of the Performance of the Decipher Genomic Classifier in High-Risk Men After Prostatectomy to Predict Development of Metastatic Disease. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. Jun 20 2017;35(18):1991-1998.
- Dalela D, Santiago-Jimenez M, Yousefi K, et al. Genomic Classifier Augments the Role of Pathological Features in Identifying Optimal Candidates for Adjuvant Radiation Therapy in Patients With Prostate Cancer: Development and Internal Validation of a Multivariable Prognostic Model. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. Jun 20 2017;35(18):1982-1990.
- Karnes RJ, Sharma V, Choeurng V, et al. Development and Validation of a Prostate Cancer Genomic Signature that Predicts Early ADT Treatment Response Following Radical Prostatectomy. Clinical cancer research : an official journal of the American Association for Cancer Research. Aug 15 2018;24(16):3908-3916.
- Yaxley JW, Raveenthiran S, Nouhaud FX, et al. Outcomes of Primary Lymph Node Staging of Intermediate and High Risk Prostate Cancer with (68)Ga-PSMA Positron Emission Tomography/Computerized Tomography Compared to Histological Correlation of Pelvic Lymph Node Pathology. The Journal of urology. Apr 2019;201(4):815-820.
- Joshi SS, Handorf ER, Sienko D, et al. Treatment Facility Volume and Survival in Patients with Advanced Prostate Cancer. European Urology Oncology.


