(UroToday.com) The 2022 American Urological Association (AUA) Annual Meeting included a session on advanced prostate cancer and a presentation by Dr. Dongho Shin discussing preliminary results of the initial experience of [177Lu] Ludotadipep treatment in patients with [18F] PSMA PET CT positive metastatic castration-resistant prostate cancer (mCRPC). Lutetium-177 labeled PSMA radio ligand therapy ([177Lu] Ludotadipep), which enables targeted delivery of beta-particle radiation to prostate cancer, has been suggested as a promising novel systemic radionuclide therapy in patients with mCRPC. At AUA 2022, Dr. Shin and colleagues highlighted their phase I trial data.
From November 2020 to August 2021, 22 men with mCRPC whom disease progressed after standard treatments were recruited for screening and eligible for treatment. Patients underwent a Florastamin labelled [18F] PSMA PET CT for screening to confirm high PSMA-expression. Five to 6 different patients were treated with [177Lu] Ludotadipep at doses of 50 mCi, 75 mCi, 100 mCi, and 125 mCi each. [177Lu] Ludotadipep was injected via venous injection and they were hospitalized for 3 days after the injection to monitoring for any adverse effects. To evaluate the treatment outcome, serum PSA levels were followed up in 1st, 2nd, 3rd, 4th, 6th, 8th, 12th weeks and [18F] PSMA PET CT was taken in 4th and 8th weeks. The primary endpoint for this study was dose limiting toxicity.
All patients showed [18F] PSMA PET CT positive in pre [177Lu] Ludotadipep treatment. The mean age was 73.2 +/- 7.6 years, with a mean PSA at trial entry of 359.4 +/- 110.0. The most common pre-trial treatment was abiraterone (27%), with a summary of the patient characteristics as follows:
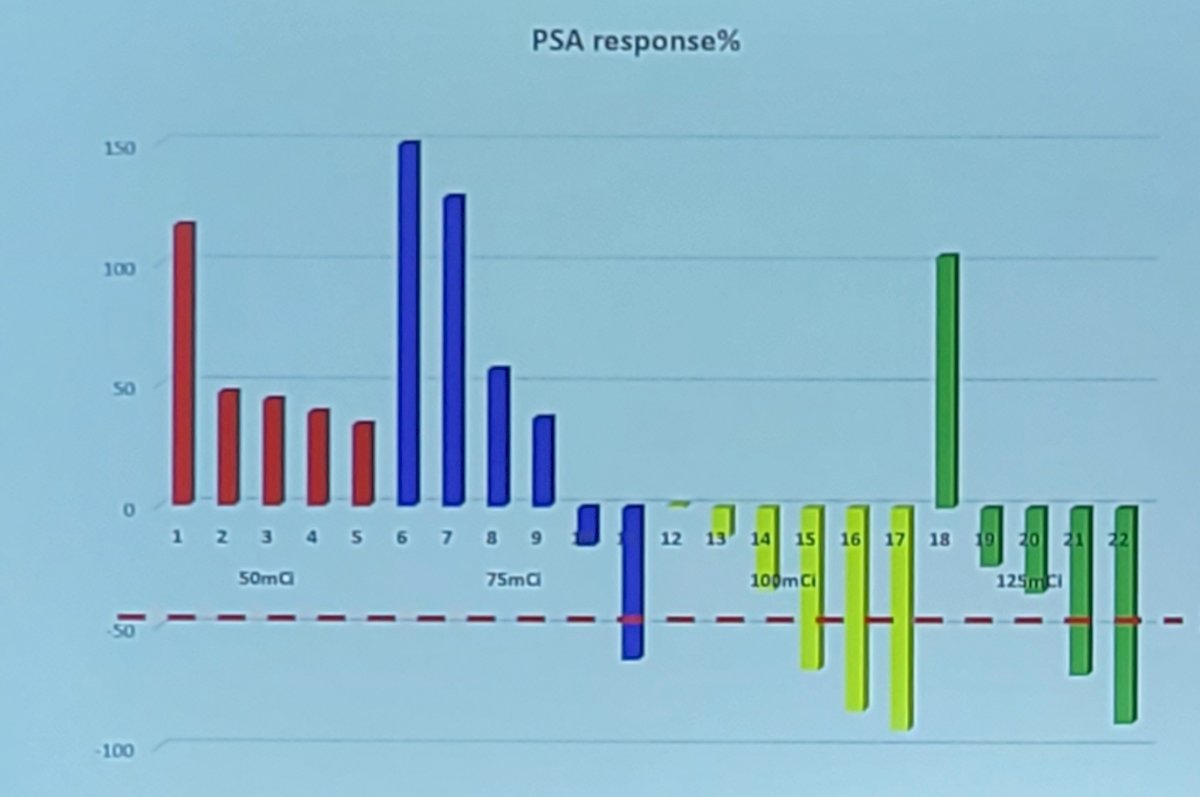
In 50 mCi treated group, 40% of patients achieved PSA partial response and non-progressive disease was seen on [18F] PSMA PET CT in 60% of patients. In 75 mCi treated group, 33.3% of patients achieved PSA partial response and non-progressive disease was seen on [18F] PSMA PET CT in 100% of patients. In 100 mCi treated group, 33.3% of patients achieved PSA partial response and rest of the patient had non-progressive disease on [18F] PSMA PET CT. As follows is the waterfall plot for PSA response:
As follows are representative images of a patient treated with [177Lu] Ludotadipep (baseline PSA 24 ng/mL0) and the subsequent follow up scan 8 weeks later (PSA 0.85 ng/mL):

With regards to safety, 6 patients complained of transient nausea (any grade) and 11 patients had any grade parotitis. There was no grade 3 or higher adverse events. Dr. Shin highlighted that a phase 2 trial is being planned with the dose of 100 mCi x 6 cycles.
Dr. Shin concluded his presentation by discussing preliminary results of the initial experience of [177Lu] Ludotadipep treatment in patients with [18F] PSMA PET CT positive mCRPC with the following take-home messages:
- The current study is in the early stages of [177Lu] Ludotadipep treatment, with a final target capacity of 150 mCi
- [177Lu] Ludotadipep could be a promising treatment with less toxicity for mCRPC patients who have not been responsive to conventional treatments
Presented By: Dongho Shin, MD, St. Mary’s Hospital, Seoul, Republic of Korea
Co-Authors: Chang Eil Yoon, Hyeok Jae Kwon, Hyong Woo Moon, Yong Hyun Park, Woong Jin Bae, Hyuk Jin Cho, U-syn Ha, Sung-Hoo Hong, Sae Woong Kim, Ji Youl Lee, Seoul, Korea, Republic of
Written By: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2022 American Urological Association (AUA) Annual Meeting, New Orleans, LA, Fri, May 13 – Mon, May 16, 2022.


