(UroToday.com) In a moderated poster presentation at the 2022 American Urologic Association Annual Meeting held in New Orleans and virtually, Dr. Jones presented on the long-term safety of darolutamide among patients with metastatic castration-resistant Prostate Cancer (mCRPC). Darolutamide (DARO) is a structurally distinct and highly potent androgen receptor inhibitor that has now been assessed in a number of advanced disease states in prostate cancer. It has demonstrated a consistently favorable safety and tolerability profile in previously reported phase 1/2 studies as well as in the phase 3 ARAMIS trial. In this analysis, the authors examined the long-term safety with extended treatment of darolutamide for more than 4 years in patients with metastatic castration-resistant prostate cancer (mCRPC) from the phase 1 ARAFOR study (NCT01784757).
This phase I, 2-part, multicenter, international study evaluated the pharmacokinetics of darolutamide in a 3-period crossover study that was followed by an open-label extension to assess long-term safety and tolerability. In this presentation, the authors provide descriptive results of patient characteristics, treatment duration, and adverse events (AEs).
Among 30 patients enrolled in ARAFOR, 6 received more than 4 years of darolutamide treatment at 600 mg twice daily. The median age was 69 years (range 58–73) and all patients were Caucasian. The median duration of treatment in ARAFOR was 63 months (range 49–90) and 1 patient completed ARAFOR and entered the darolutamide roll-over study (NCT04464226).
Among the six patients who received more than 4 years of darolutamide, at baseline, five patients had normal renal function while 1 patient having mild renal impairment. Three patients had normal hepatic function and 3 had mild hepatic impairment. Eastern Cooperative Oncology Group performance status was 0 for 5 patients and 1 for 1 patient. No patient received prior chemotherapy.
All 6 patients reported adverse events with 3 patients reporting diarrhea, and 2 patients each reporting abdominal pain, nausea, arthralgia, hematuria, influenza, anemia, dysuria, fall/accident, rib fracture, solar dermatitis, and decreased weight. Among these, grade 3 adverse events occurred in 5 patients and serious adverse events occurred in 4 patients. However, no individual grade 3 or serious adverse event occurred in more than 1 patient and none were judged to be related to darolutamide. Treatment-related adverse events (tinnitus, fatigue, solar dermatitis) were reported in 2 patients and were grade 1 in worst severity.
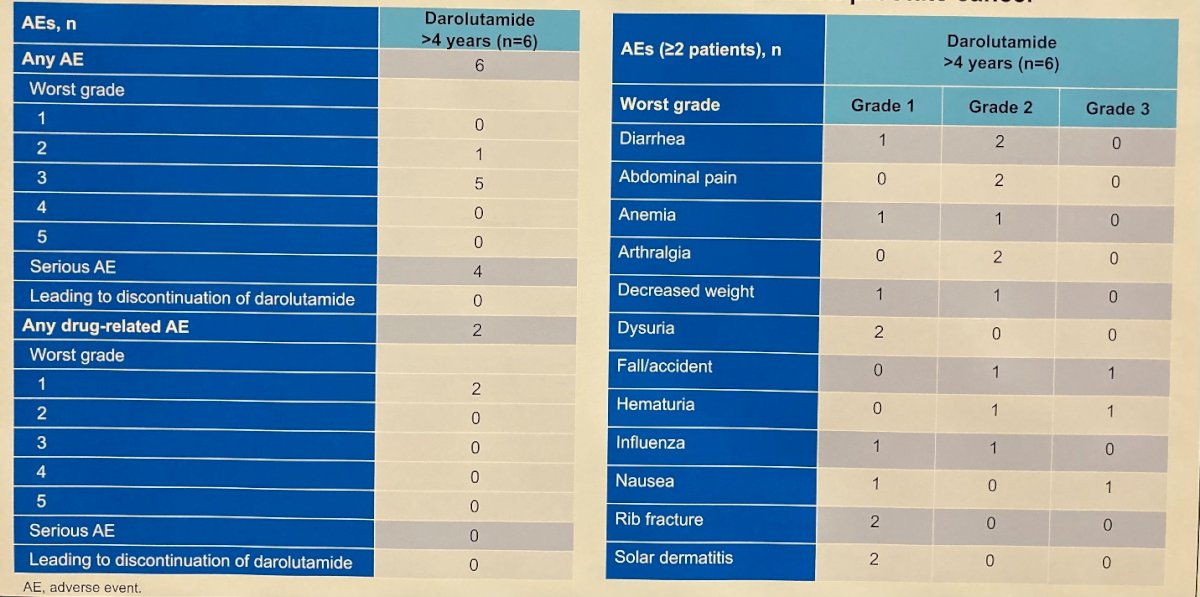
No patients discontinued darolutamide due to adverse events.
Thus, the authors concluded that, in this small group of patients with mCRPC from the phase 1 ARAFOR study, long-term treatment with darolutamide beyond 4 years was generally well tolerated and no new safety signals were observed.
Presented by: Robert Jones, MD,PhD, Professor of Medical Oncology, Cardiff University and Velindre Cancer Centre


