The KEYNOTE-045 was a phase 3 trial comparing pembrolizumab vs. investigator’s choice (paclitaxel, docetaxel, or vinflunine) in 2nd line recurrent, advanced urothelial carcinoma. This showed a clear benefit to pembrolizumb (Figure 1). In contrary, the IMVIGOR 211 trial comparing atezolizumab to chemotherapy in platinum-treated metastatic urothelial carcinoma, was a negative trial.
Figure 1 – KEYNOTE 045 trial comparing pembrolizumab to investigator’s choice:
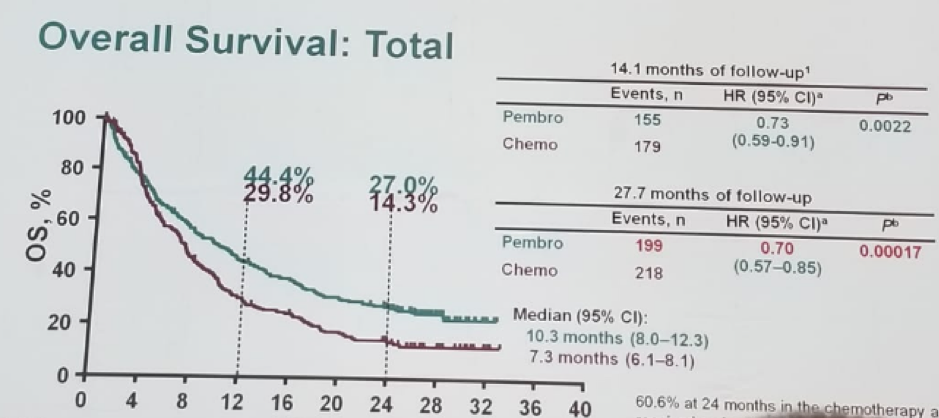
According to Dr. Leibowitz, the single agent anti-PD1/PD-L1 is on the verge of being statistically significant as 2nd line therapy in platinum-refractory urothelial cancer in unselected patients. PD-1 expression is a biomarker for response to this class of agents (although the dilemma on how to assess this is a whole other conundrum).
Dr. Leibowitz moved on to discuss the role of anti-PD1/L1 antibodies in the 1st line therapy for metastatic urothelial carcinoma. According to her, the jury is still out in this specific scenario. The data on the inferiority of carboplatin vs. cisplatin is historical, retrospective, based on very small cohorts and biased. A true prospective comparison has never been performed. Logic has it that these drugs are very similar if not identical. Single-agent anti-PD1/L1 has recently been called by the FDA (May 2018) to be inferior and dangerous to patients with PD-1 negative compared to a carbo-based regimen, in two independent trials. Single-agent anti PD1 has not yet been proven to be superior (or even identical) to any chemotherapy in anti-PD1 positive patients in 1st line therapy. In an unselected patient population, most patients do not respond to single-agent anti-PD1-L1 antibodies. Even within responders, it seems that there are no long-term remissions nor cures. PD1/L1 expression, in a manner yet to be defined and calibrated, is a biomarker of response across all lines. It is important to remember that significant, life-threatening toxicities can occur. Rapid disease progression prior achievement of response is a major concern. All patients with upper tract TCC, and patients with bladder cancer and a suggestive family history, should be tested for Lynch syndrome.
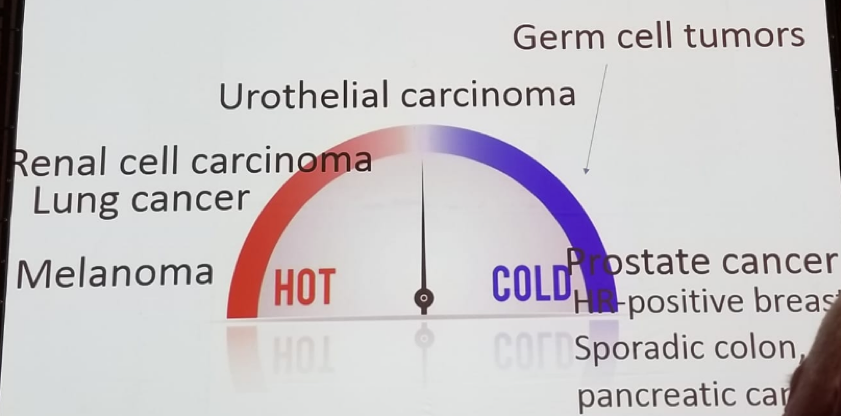
Dr. Leibowitz continued and discussed the “race” to make “cold” tumors “hot” (Figure 2). As opposed to other “hot” malignancies (melanoma, lung cancer, renal cell cancer), urothelial carcinoma is in between hot and cold (Figure 3).
Figure 2 – The race to make “cold” tumors “hot”:
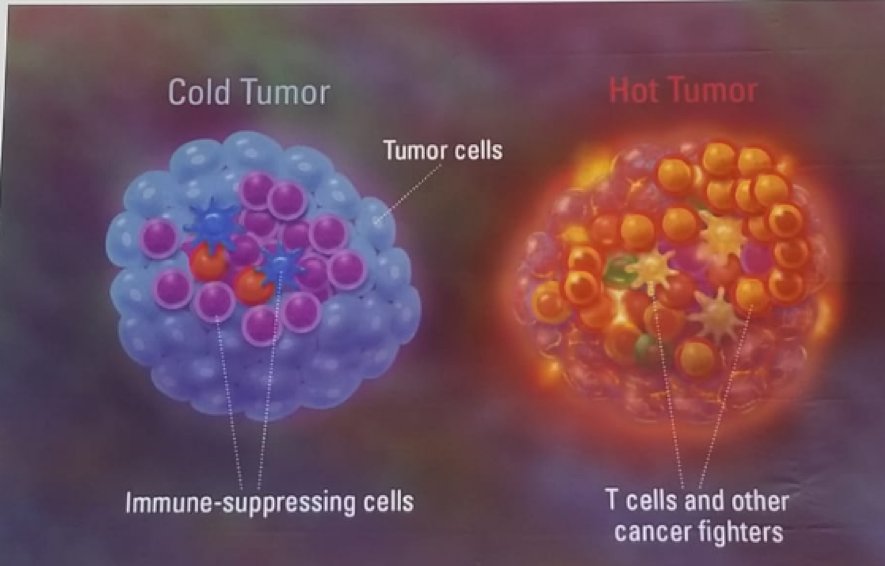
Figure 3 – The ‘temperature’ of Genitourinary cancers:
Dr. Leibowitz concluded her great talk stating there is still a long way to go before immunotherapy can become the mainstay of treatment across the full spectrum of genitourinary cancers. Biomarkers for response/progression are desperately needed. Lastly, analysis of the checkpoint networks can highlight potential new combinations for clinical studies.
Presented by: Raya Leibowitz-Amit, MD, PhD, Sheba Medical Center, Israel
Written by: Hanan Goldberg, MD, Urologic Oncology Fellow (SUO), University of Toronto, Princess Margaret Cancer Centre @GoldbergHanan at the 2018 FOIU 4th Friends of Israel Urological Symposium, July 3-5. 2018, Tel-Aviv, Israel


