- First line (cisplatin-ineligible patients) - Atezolizumab and Pembrolizumab
- Platinum pretreated - Atezolizumab, Nivolumab, Durvalumab, Avelumab, and Pembrolizumab.
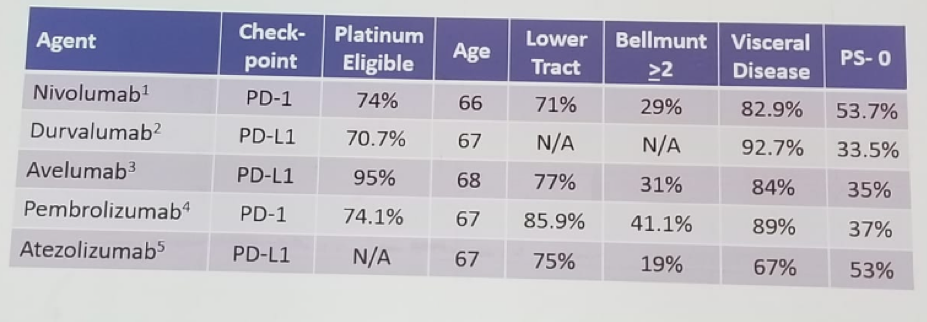
Table 1 – Immune checkpoint inhibitors in the platinum-refractory setting:

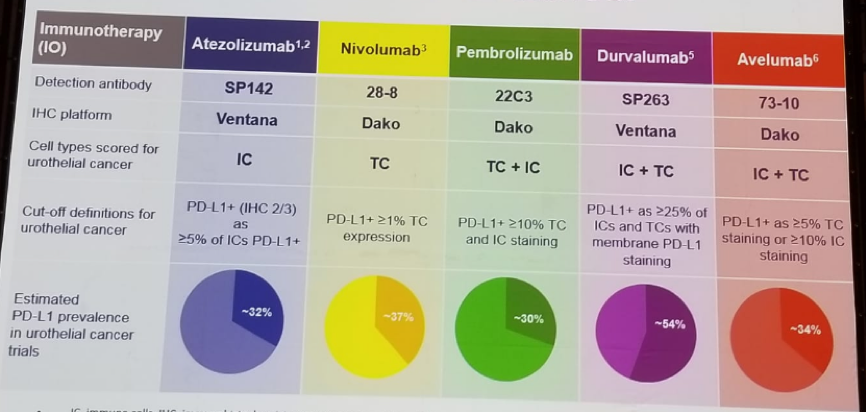
Table 2 –Assays for PD-L1 expression in urothelial cancer:
In May 2018 the FDA issued an alert as two ongoing clinical trials (IMVIGOR-130, and keynote-361) found that in patients with monotherapy ICI (pembrolizumab or Atezolizumab) with PD-L1 low status, there was decreased survival compared to patients who received cisplatin or carboplatin-based chemotherapy. Therefore, both trials have stopped enrolling patients whose tumors have PD-L1 low status to the pembrolizumab or atezolizumab monotherapy arms. The monotherapy arms remain open only to patients whose tumors have high PD-L1 status.
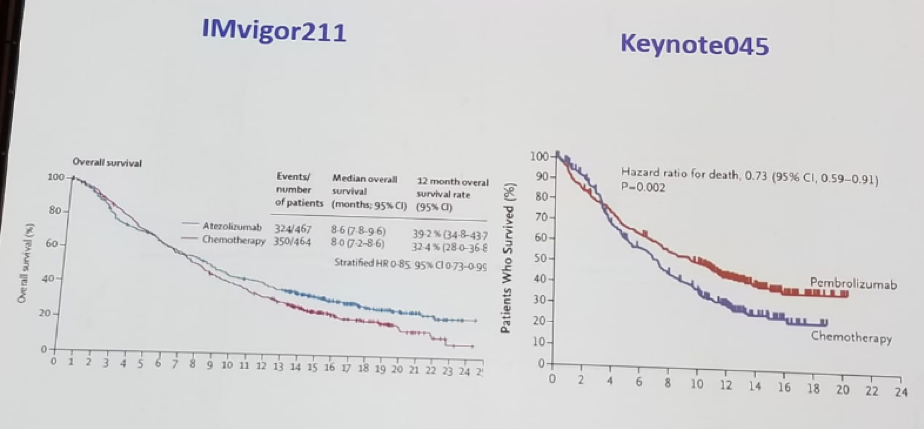
Table 3 demonstrates ICI in 2nd line metastatic urothelial carcinoma. In the KEYNOTE-045 trial patients after 2nd line platinum-based chemotherapy were randomized to either pembrolizumab or chemotherapy with docetaxel, paclitaxel, and vinflunine. At 24 months 60.6% of the chemotherapy arm received a ICI agent, including those who received pembrolizumab as part of the crossover. At the median follow-up time, a clear overall survival (OS) advantage was seen in favor of the ICI arm with 10.3 months (8-12.3) vs. 7.3 months (6.1-8.1).
Table 3 – Immuno-oncology treatments for 2nd line -metastatic urothelial carcinoma:
In the IMVIGOR211 study metastatic urothelial carcinoma patients after 2nd line, platinum-based chemotherapy was randomized to either atezolizumab or chemotherapy (vinflunine, paclitaxel, docetaxel). OS was better with atezolizumab (8.6 vs. 8 months). In the study, it was shown that PD-L1 status was prognostic but not predictive. The OS in these phase 3 trials are shown in figure 1.
Figure 1 - Comparison of survival in the phase 3 trials:
Second line immunotherapy has an objective response rate of 20% with a median of 8-18 months. There is improved OS with Pembrolizumab compared to chemotherapy and also an improved toxicity compared to chemotherapy. The PDL1 expression is not needed. There is no ascertainable difference in clinical activity between PDL1 and PD1 inhibitors. Progression-free survival was 2-3.3 months.
Sella continued to discuss some unresolved issues with immunotherapy. The first question raised was when to stop therapy in responding patients, how to integrate surgery for residual disease and how to define progression. Lastly, Toxicity is an issue as well, as the spectrum of toxicity in immunotherapy is quite vast, including mainly rash, pruritus, liver toxicity, diarrhea, colitis, and hypophysitis. These occur in more than 10% of patients.
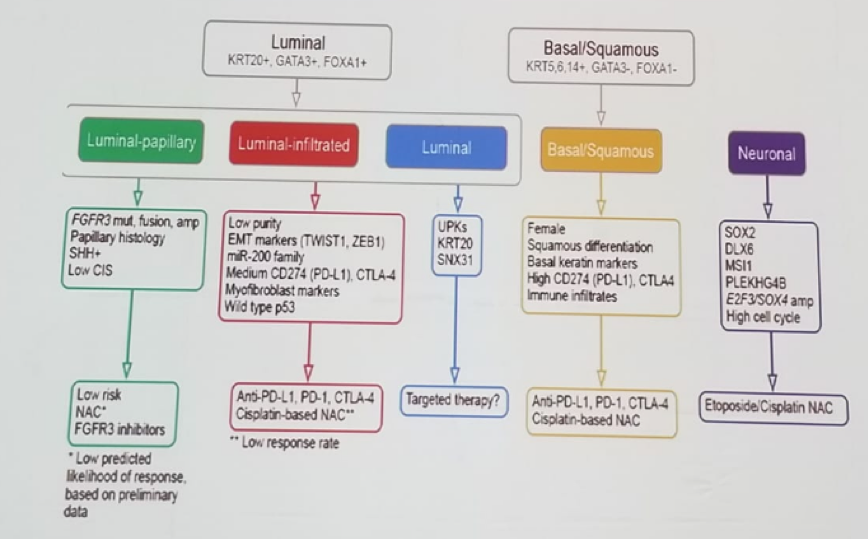
Combination immunotherapies was the last topic discussed by Sella. The combination of Nivolumab and Ipilumab in Checkmate 032 trial was mentioned and also the combination of Durvalumab and Tremelimumab. Tumor mutational burden is associated with a higher objective response rate. The response rate is also affected by the TCGA molecular subtype (Luminal papillary, luminal infiltrated, luminal, basal squamous, and neuronal), as seen in Figure 2.
Figure 2 – Treatment selection by molecular subtype:
Sella concluded his talk by stating that in the future we will use multiple combination therapies, precision therapy, and without any formal lines of therapy, as we currently utilize. Obviously, much more research is needed to fully understand immunotherapy.
Presented by: Avishay Sella, MD, Asaf Harofe Medical Center, Israel
Written by: Hanan Goldberg, MD, Urologic Oncology Fellow (SUO), University of Toronto, Princess Margaret Cancer Centre @GoldbergHanan at the 2018 FOIU 4th Friends of Israel Urological Symposium, July 3-5. 2018, Tel-Aviv, Israel


