(UroToday.com) The 2022 EAU annual meeting featured a joint session of the EAU, EANM, ESMO, and ESTRO societies examining modern diagnostic and therapeutic approaches in prostate cancer, including a presentation by Dr. Ken Herrmann discussing future perspectives for possible synergies between radioligand and systemic treatment in M1 prostate cancer. Dr. Herrmann notes that synergism of treatment is the holy grail, emphasizing that in the VISION trial 46.0% of patients had a >= 50% confirmed decrease in disease, and 33.0% of patients had a >= 80% decrease. Dr. Herrmann highlights that the difference between additive and synergistic therapy is as follows:
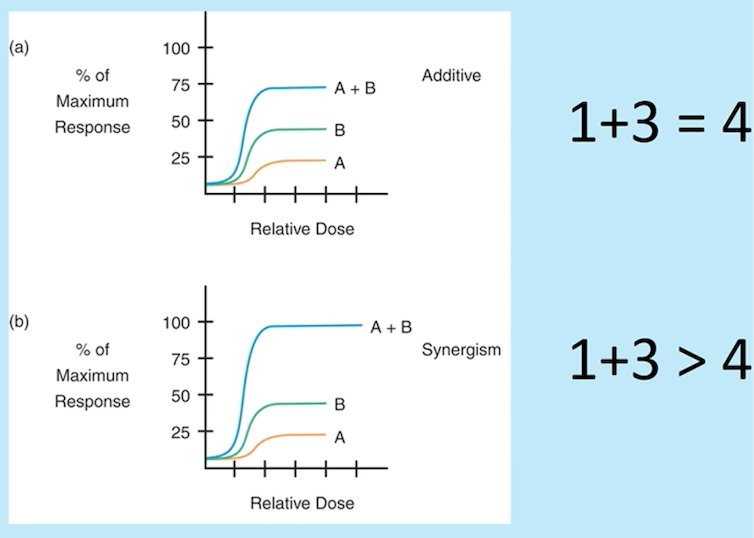
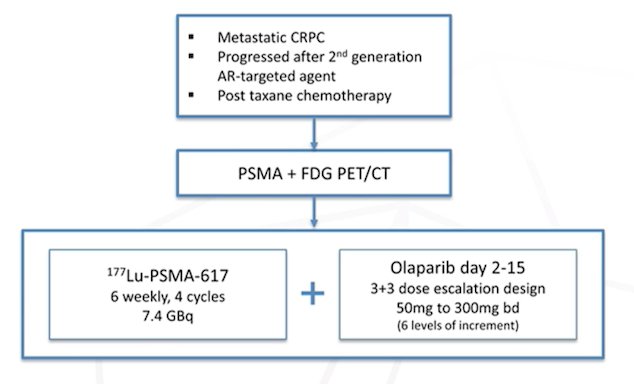
There are several candidates for potential synergistic combinations, including (i) checkpoint inhibitors, (ii) PARP inhibitors, (iii) androgen receptor directed therapy, (iv) chemotherapy, (v) SBRT, and (vi) potentially others. Dr. Herrmann then highlighted several trials that are assessing synergistic combination therapy. The first trial he discussed is assessing PSMA radioligand therapy + pembrolizumab in men with mCRPC (n = 37), with an update of this trial at ASCO 2022 showing a PSA >= 50% response of 76% (95% CI 59-88%) and an objective response rate by RECIST 1.1 of 78% (7/9 patients). With regards to PARP inhibitors, the LuPARP trial is a phase 1 trial of 177Lu-PSMA-617 therapy + olaparib among men with mCRPC that have progressed after 2nd generation androgen receptor-targeted therapy and taxane chemotherapy:
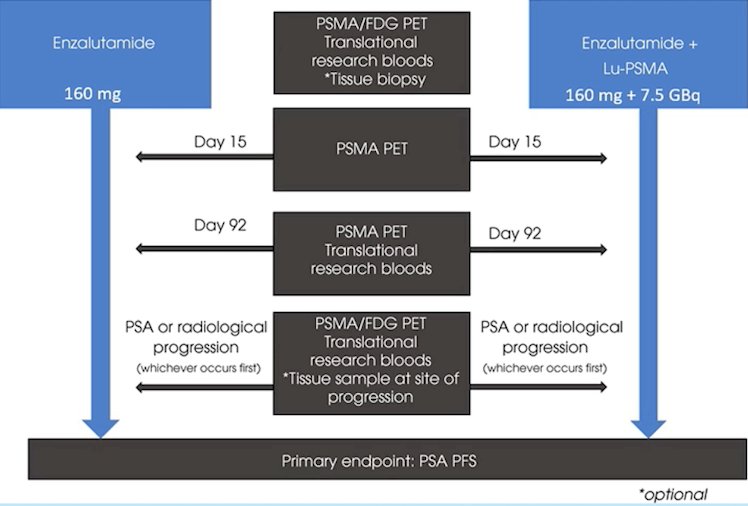
Two trials are assessing androgen receptor directed therapies. The ENZA-p trial is testing enzalutamide + Lu-PSMA versus enzalutamide with a primary endpoint of PSA progression free survival:
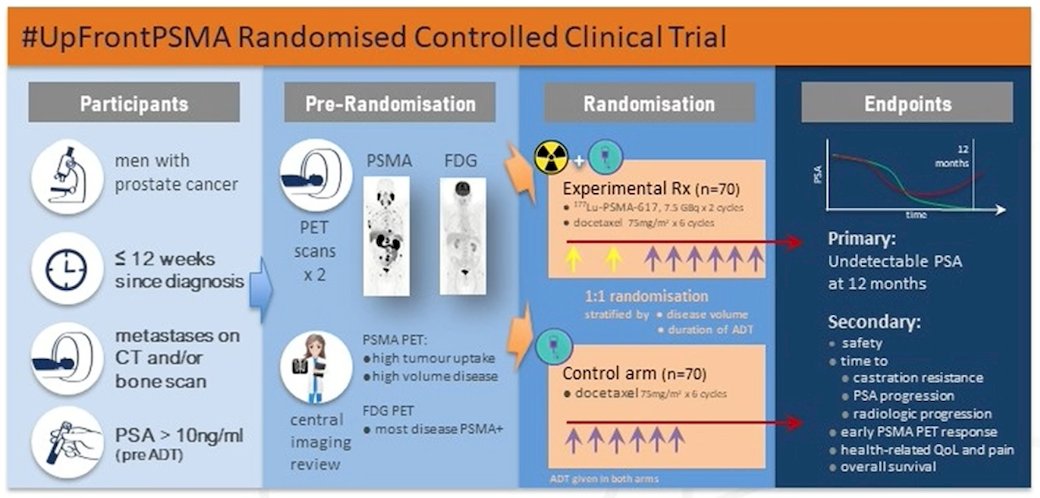
The PSMAddition trial is a randomized, phase 3 study of 177Lu-PSMA-617 in patients with untreated or minimally treated metastatic hormone sensitive prostate cancer. These men will be randomized to 177Lu-PSMA-617 + standard of care versus standard of care with a primary endpoint of radiographic progression free survival. The UpfrontPSMA (NCT04343885) trial will enroll men with prostate cancer < 12 weeks since diagnosis, with metastases on CT and/or bone scan, and PSA >10 ng/mL before ADT. Prior to randomization they will undergo PSMA and FDG PET/CT, followed by randomization to 177Lu-PSMA-617 + docetaxel (n = 70) versus docetaxel (n = 70) and a primary end point of undetectable PSA at 12 months:
SBRT is also being assessed in combination with 177Lu-PSMA-617 given that the rationale of adding SBRT in patients with few, disease dominant but PSMA-negative or PSMA-low uptake lesions, is very appealing. A phase 1 trial at Memorial Sloan Kettering Cancer Center is ongoing with an estimated enrollment of 6 patients. Finally, Dr. Herrmann notes that there are several other current Lu-PSMA combination trials that are ongoing, as highlighted in the following figure:
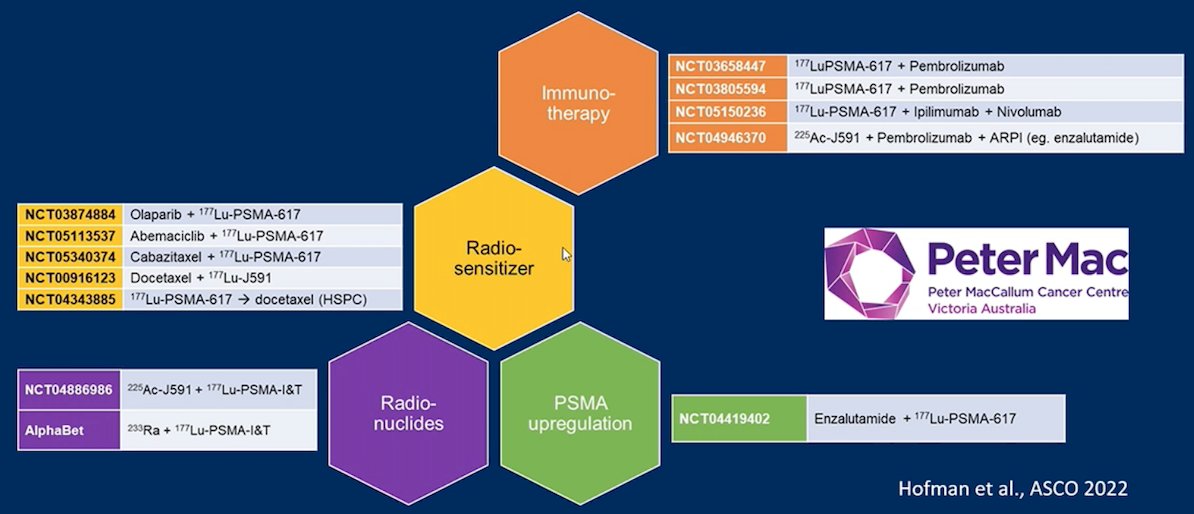
Dr. Herrmann concluded his presentation by discussing future perspectives for possible synergies between radioligand and systemic treatment in M1 prostate cancer with the following take-home messages:
- Aiming for synergism remains the holy grail of advanced prostate cancer
- Early indicators for potential synergism are there, particularly with checkpoint inhibitors and androgen receptor-directed therapy
- The ‘hit hard, hit early’ strategy is not undisputed
- New radioligand concepts are underway
Presented by: Ken Herrmann, MD, MBA, Department of Nuclear Medicine, University of Duisburg-Essen and German Cancer Consortium – University Hospital Essen, Essen, Germany
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2022 European Association of Urology (EAU) Annual Hybrid Meeting, Amsterdam, NL, Fri, July 1 – Mon, July 4, 2022.
References:


