- Apalutamide, an oral nonsteroidal androgen receptor inhibitor, was approved by the US Food and Drug Administration and the European Medicines Agency for nonmetastatic castration-resistant prostate cancer (nmCRPC). Approval was based on results of the pivotal phase 3, placebo-controlled, international SPARTAN study in 1207 patients at high risk of developing metastasis who were receiving ongoing androgen deprivation therapy (ADT).1-3
- In the primary analysis of SPARTAN, as reported in The New England Journal of Medicine, the addition of apalutamide to ADT extended metastasis-free survival (MFS; the primary endpoint) by greater than 2 years without compromising health-related quality of life (HRQoL).2,4
- Based on these data, SPARTAN was unblinded and all patients who had not progressed who were actively receiving placebo (n = 76; 19% of the entire placebo group) crossed over to the apalutamide group.2,5
- At the time of primary analysis, with 20.3 months of median follow-up, overall survival (OS) data were immature2; therefore, it was essential to evaluate OS at the time of final analysis of SPARTAN.
FINAL ANALYSIS OF SPARTAN
Overall Survival
- In the final analysis of SPARTAN, with median 52 months of follow-up, as reported in European Urology, apalutamide significantly extended the median OS (a secondary endpoint) by 14.0 months and was associated with a 22% reduction in risk of death compared with placebo (Figure 1); the treatment effect was generally consistent across study subpopulations.6
- The benefit is even more impressive given that 19% of placebo-treated patients crossed over during the study and a total of 84% of the patients who received placebo received subsequent life-prolonging therapy.5,6
- Moreover, when the placebo to apalutamide crossover group was censored using either of two independent exploratory sensitivity analyses, the median OS difference increased from 14 months to 21 months between the apalutamide and placebo groups.6
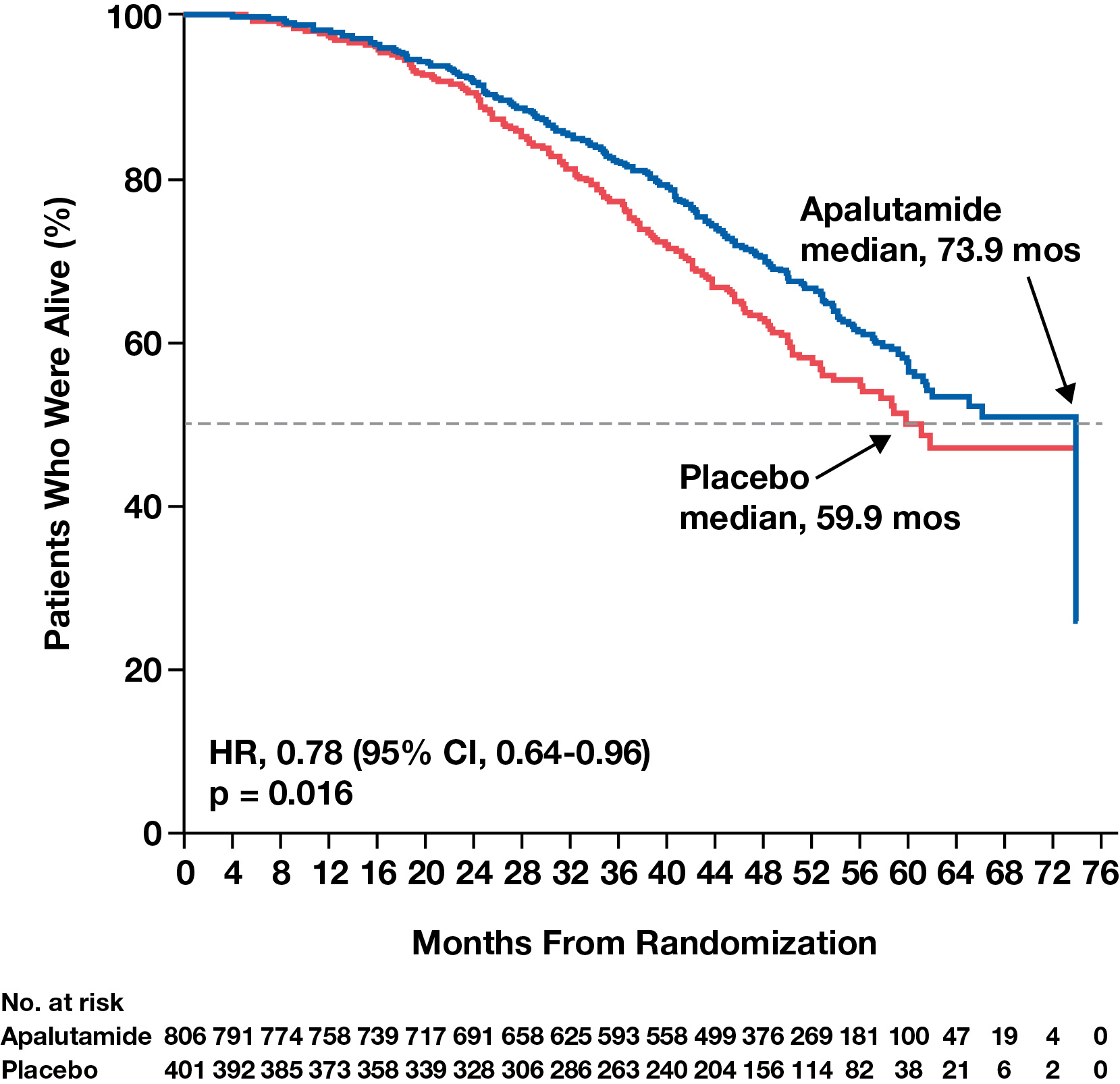
Figure 1. Apalutamide significantly increased median OS in patients with nmCRPC compared with placebo.6
Reprinted with permission. This figure was published in European Urology, Volume 79, Smith MR, Saad F, Chowdhury S, et al. Apalutamide and Overall Survival in Prostate Cancer. Pages 150-158. Copyright © Elsevier (2020).
Second Progression-Free Survival
- Additionally, at final analysis, apalutamide was shown to extend second progression-free survival (PFS2; an exploratory endpoint of SPARTAN) by 14.4 months compared with placebo (Figure 2).6
- PFS2 was defined as the time from randomization to investigator-assessed disease progression (by prostate-specific antigen level, imaging, or symptom development) during or after the first subsequent treatment, or death from any cause, whichever occurred first.6
- Benefit in PFS2, coupled with apalutamide benefit in MFS (Figure 3), is an indicator of effective early intensification of treatment.
- Benefit in PFS2 is also consistent with apalutamide OS benefit.
- Together, these data support earlier treatment with apalutamide.2,6
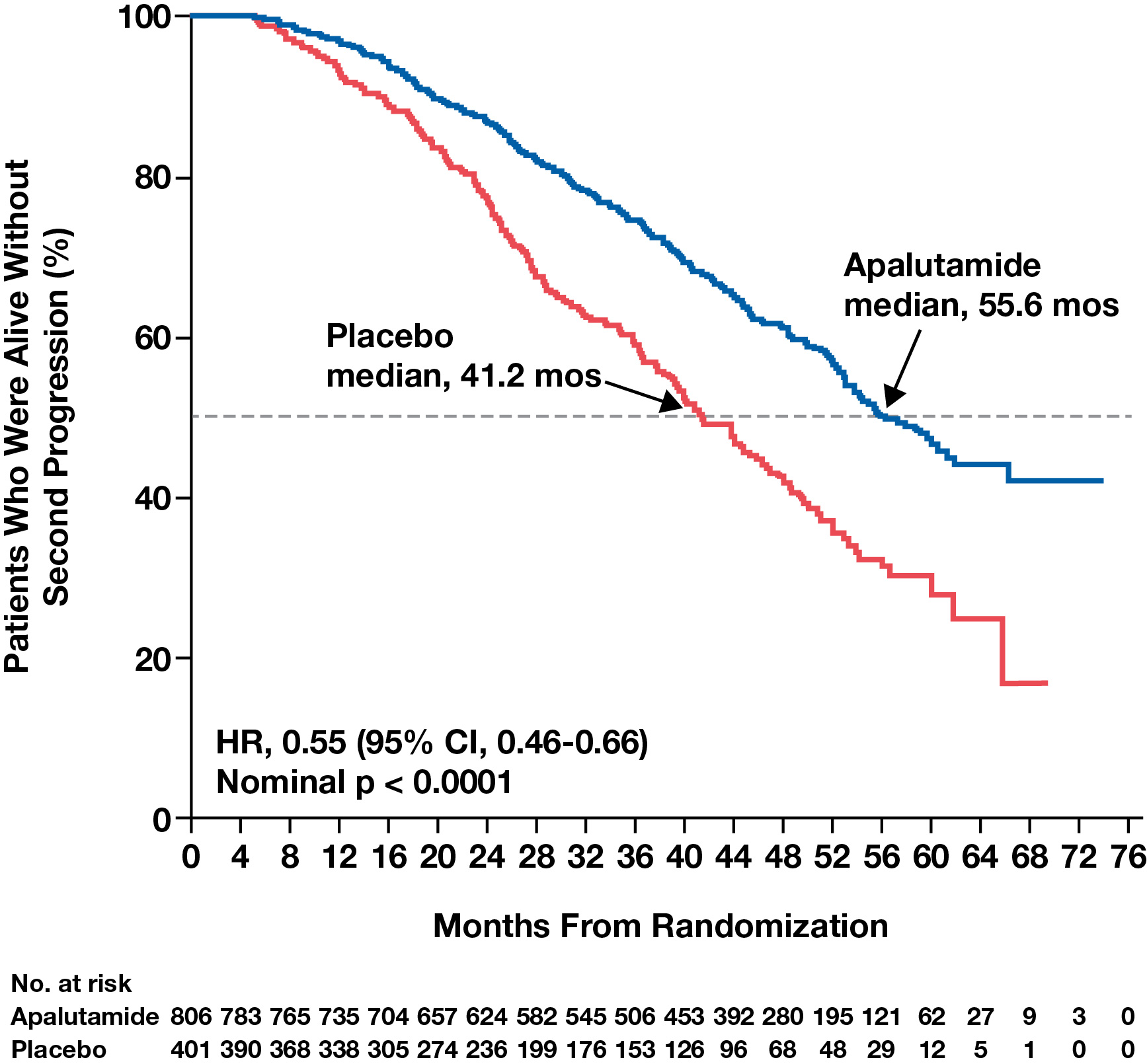
Figure 2. Apalutamide extended PFS2 in patients with nmCRPC compared with placebo.6
Reprinted with permission. This figure was published in European Urology, Volume 79, Smith MR, Saad F, Chowdhury S, et al. Apalutamide and Overall Survival in Prostate Cancer. Pages 150-158. Copyright © Elsevier (2020).
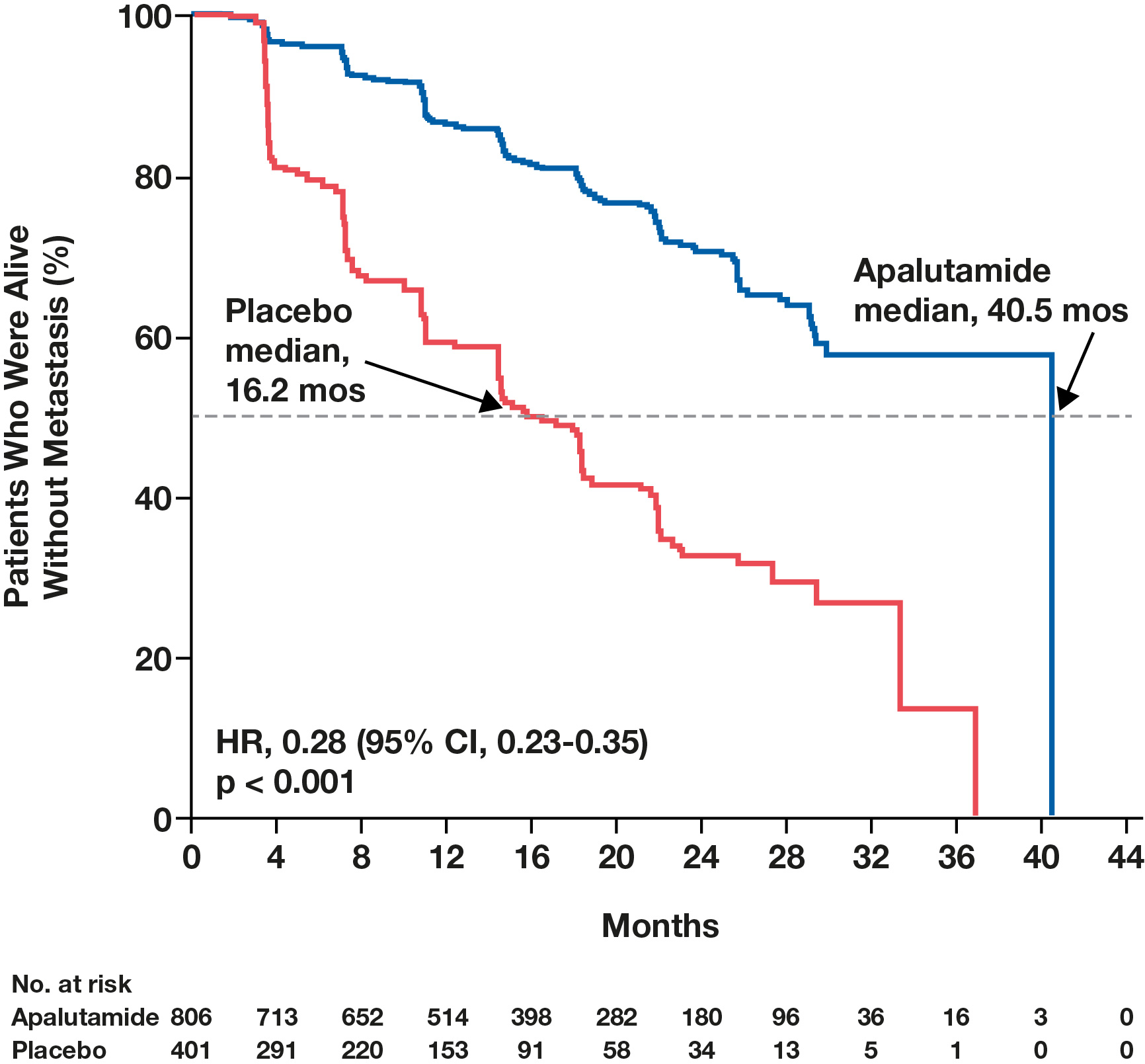
Figure 3. Apalutamide significantly increased MFS in patients with nmCRPC compared with placebo.2
From The New England Journal of Medicine, Smith MR, et al. Apalutamide treatment and metastasis-free survival in prostate cancer 2018;378(15):1408-1418. Copyright © (2018) Massachusetts Medical Society. Reprinted with permission.
Retrospective Analysis
- Retrospective analysis between MFS and OS concluded that development of metastasis in patients with nmCRPC, regardless of time point, is associated with a significantly greater hazard of death and, therefore, that MFS is predictive of OS.2,7
Safety
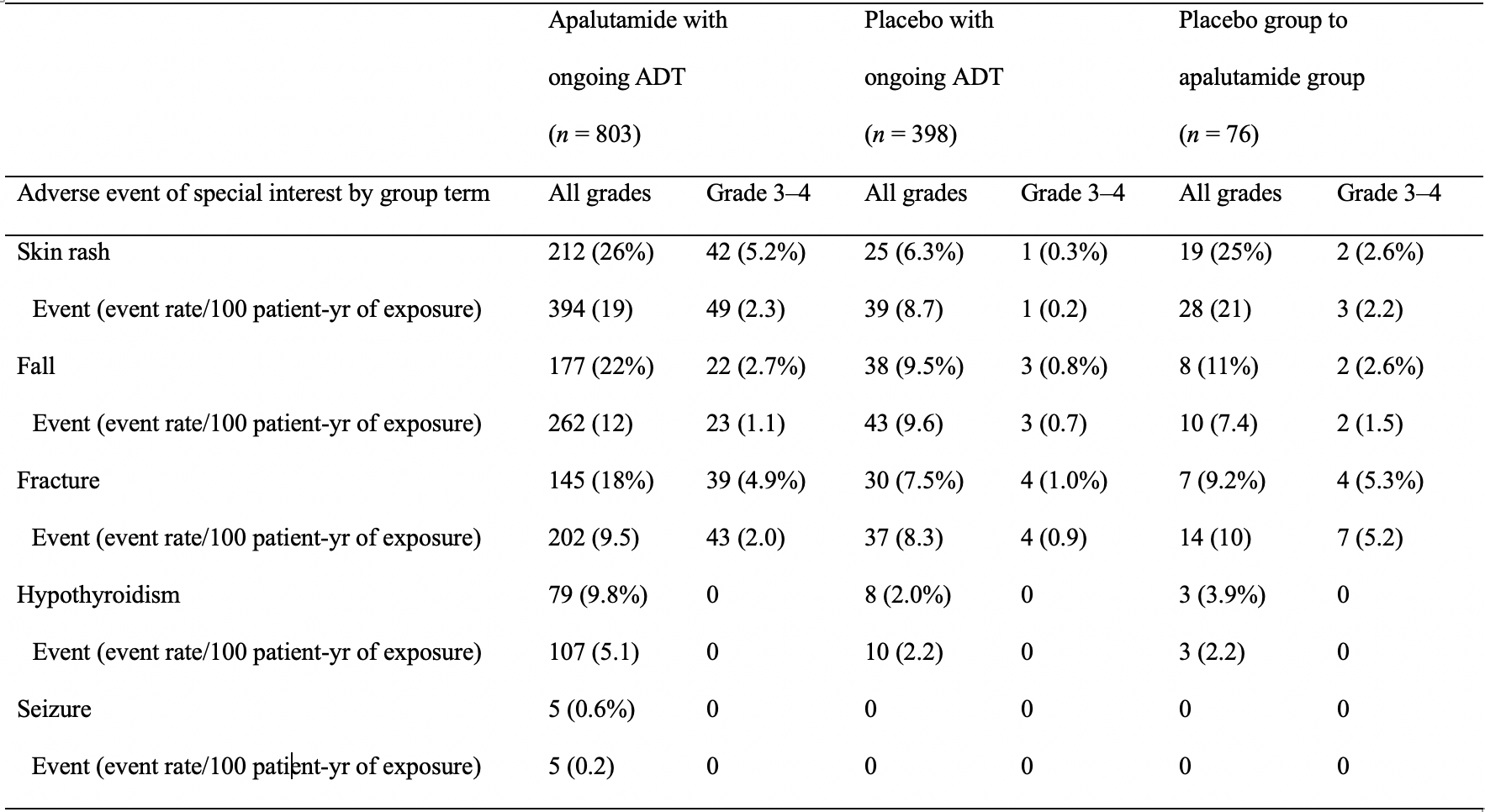
- At 52 months of median follow-up, the safety profile of apalutamide remained consistent with that of previous reports.6
- Although the overall median treatment duration for patients in the apalutamide group was
4 months longer than in the placebo group (apalutamide, 32.9 months; placebo 11.5 months), no adverse event or serious adverse event rates had a notable increase with prolonged apalutamide exposure (Table 1). - Adverse and serious adverse event rates had no notable increase with longer apalutamide exposure/follow-up.6
Table 1. Treatment-emergent AEs of special interest and exposure-adjusted rates/100 patient-yr.6
Reprinted with permission. This table was published in European Urology, Volume 79, Smith MR, Saad F, Chowdhury S, et al. Apalutamide and Overall Survival in Prostate Cancer. Pages 150-158. Copyright © Elsevier (2020).
Patient-Reported Outcomes
- Patient-reported outcomes (PROs) were prespecified exploratory end points for the SPARTAN study to evaluate health-related quality of life (HRQoL), and PROs at the time of the primary analysis have been reported.4
- Updated PROs from the final analysis of SPARTAN, with 52 months of median follow-up, were reported at the European Society for Medical Oncology (ESMO) 2020 virtual conference.8
- To measure PROs, the Functional Assessment of Cancer Therapy-Prostate (FACT-P) and EuroQoL 5-dimension, 3-level questionnaire (EQ-5D-3L) instruments were administered at baseline, frequently during treatment, and after disease progression, to provide an in-depth understanding of the patient experience.
- At final analysis, patients who received apalutamide in addition to ADT maintained stable overall HRQoL over time, while scores in the placebo plus ADT group tended to decline after approximately 1 year.
- Most patients indicated that they were not at all bothered by side effects of apalutamide, and bother did not increase over time.
- Of note, based on the individual FACT-P question GP1, “I have a lack of energy,” fatigue did not increase over time with addition of apalutamide to ADT.8
- These results are important because fatigue can be debilitating for patients with cancer9; it is essential that the fatigue patients are already experiencing from their cancer and ADT treatment is not exacerbated with the addition apalutamide.
Summary
- In conclusion, results from the final analysis of SPARTAN demonstrated that the addition of apalutamide to ADT provides a significant and clinically meaningful improvement in OS while maintaining HRQoL in patients with nmCRPC.
- After 52 months of median follow-up, the longest median follow-up reported among the phase 3 studies of androgen signaling inhibitors for patients with nmCRPC (ie, compared with median follow-up of 29.0 months in the ARAMIS study of darolutamide10 and 48.0 months in the PROSPER study of enzalutamide11), the safety profile of apalutamide remained consistent with previous reports.
- The totality of data from the SPARTAN study supports apalutamide plus ADT as a highly effective, tolerable treatment that significantly improves MFS, PFS2, and OS, while preserving HRQoL for patients with nmCRPC.
Acknowledgments
Editorial assistance was provided by William Turner, PhD, of Parexel, with funding from Janssen Global Services, LLC.
Written by: Simon Chowdhury,1 Ke Zhang,2 Peter De Porre,3 Sabine Brookman-May,4,5 Neeraj Agarwal6
- Guy’s, King’s, and St Thomas’ Hospitals, and Sarah Cannon Research Institute, London, UK
- Janssen Research & Development, San Diego, CA, USA
- Janssen Research & Development, Beerse, Belgium
- Janssen Research & Development, Spring House, PA, USA
- Ludwig Maximilian University, Munich, Germany
- Huntsman Cancer Institute, University of Utah, Salt Lake City, UT, USA
Corresponding author:
Neeraj Agarwal, MD, Huntsman Cancer Institute, University of Utah, Salt Lake City, UT
Financial Support: This clinical study was supported by Janssen Research & Development. Editorial assistance was provided by William Turner, PhD, of Parexel, with funding from Janssen Global Services, LLC.
References:
- ERLEADA (apalutamide) [prescribing information]. Janssen Pharmaceutical Companies, Horsham, PA; 2019.
- Smith MR, Saad F, Chowdhury S, et al. Apalutamide treatment and metastasis-free survival in prostate cancer. N Engl J Med. 2018;378(15):1408-1418.
- ERLEADA (apalutamide) [summary of product characteristics]. The electronic Medicines Compendium (eMC) Web site (https://www.ema.europa.eu/en/documents/product-information/erleada-epar-product-information_en.pdf) and https://www.ema.europa.eu/en/medicines/human/EPAR/erleada. Accessed November 3, 2020.
- Saad F, Cella D, Basch E, et al. Effect of apalutamide on health-related quality of life in patients with non-metastatic castration-resistant prostate cancer: an analysis of the SPARTAN randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2018;19(10):1404-1416.
- Small EJ, Saad F, Chowdhury S, et al. Apalutamide and overall survival in non-metastatic castration-resistant prostate cancer. Ann Oncol. 2019;30(11):1813-1820.
- Smith MR, Saad F, Chowdhury S, et al. Apalutamide and overall survival in prostate cancer. Eur Urol. 2021;79(1):150-158.
- Smith MR, Mehra M, Nair S, Lawson J, Small EJ. Relationship between metastasis-free survival and overall survival in patients with nonmetastatic castration-resistant prostate cancer. Clin Genitourin Cancer. 2020;18(2):e180-e189.
- Oudard S, Hadaschik BA, Saad F, et al. Health-related quality of life (HRQoL) at final analysis of the SPARTAN study of apalutamide (APA) vs placebo (PBO) in patients (pts) with non-metastatic castration-resistant prostate cancer (nmCRPC) receiving androgen deprivation therapy (ADT). Ann Oncol. 2020;31:S521; Abs 632P.
- Storey DJ, McLaren DB, Atkinson MA, et al. Clinically relevant fatigue in men with hormone-sensitive prostate cancer on long-term androgen deprivation therapy. Ann Oncol. 2012;23(6):1542-1549.
- Fizazi K, Shore N, Tammela TL, et al. Nonmetastatic, castration-resistant prostate cancer and survival with darolutamide. N Engl J Med. 2020;383(11):1040-1049.
- Sternberg CN, Fizazi K, Saad F, et al. Enzalutamide and survival in nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2020;382(23):2197-2206.
Related Content:
ASCO 2020: Final Survival Results from SPARTAN, A Phase III Study of Apalutamide versus Placebo in Patients with Nonmetastatic Castration-Resistant Prostate Cancer


