However, at one point, in a level III laboratory in the Pasteur Institute, it became evident that based on regulatory aspects, it will be almost impossible to bring these level III classified genetically modified BCGs into humans. With this in mind we started to investigate the few clinically tested recombinant BCG vaccination strains for tuberculosis and were really lucky to find an interesting candidate BCG fulfilling all our needs: Stefan Kaufmann, at that time Director of the Department of Immunology, Max Plank Institute for Infection Biology, and Leander Grode, at that time CSO of Vaccine Project Management GmbH (VPM) had developed a recombinant BCG in which they deleted an important resistance mechanism of BCG (Urease C) by introducing the hemolysine lysteriolysin (hly) from Listeria monocytogenes, a strong T-cell activator (Grode, JCI 2005; PMID: 16110326). For more details, please refer to Figure 1. The exchange of the urease C gene with the listeriolysin gene results in profound improvement of immunity of VPM1002 over BCG. Egress of antigens from VPM1002 into the cytosol of infected cells promotes a broader adoptive immune response comprising CD4 and CD8 T cells. Egress of double-stranded DNA into the cytosol induces a stronger innate immune response including transient inflammation and autophagy. Autophagy also likely contributes to more rapid elimination of VPM1002 as compared to standard BCG in the host. Because listeriolysin is only active at acidic pH and rapidly degraded in the cytosol of the host cell, its effects are short-lived and do not cause major damage. All these mechanisms contribute to a higher immunogenicity and safety of VPM1002 and made VPM1002BC an interesting candidate BCG for the treatment of NMIBC. VPM1002 was consequently reformulated for the use in bladder cancer and named VPM1002BC.
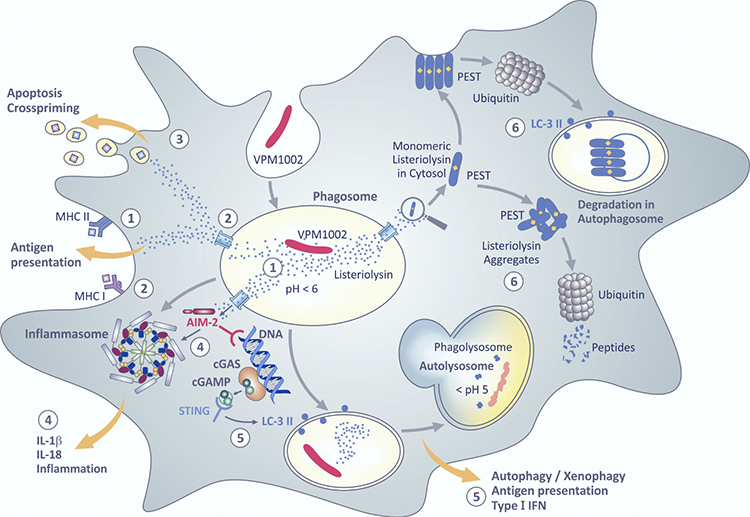
Figure 1. Proposed intracellular pathways induced by VPM1002.
Clinical trial development of VPM1002BC in bladder cancer: After 3 years of preclinical development we started, together with the Swiss Group of Clinical Cancer Research (SAKK), VPM, and one of the largest and most innovative vaccine manufacturers, Serum Institute of India, Pune, India, designing a phase I-II clinical trial in NMIBC patients previously exposed to BCG - SAKK 06/14. This has led, for the first time, to the official permissions in the Netherlands, Germany, and Switzerland allowing the use of a genetically modified BCG, VPM1002BC, for the treatment of bladder cancer in a clinical trial setting. VPM1002BC was very well tolerated as only 2/42 patients did not tolerate more than 2 intravesical applications. One and 3- year efficacy defined as recurrence-free rate in the bladder after treatment start was 49.3 and 43.7%, respectively. This is, even though the trial lacks a control arm to corroborate the result, a remarkable result in this pre-treated patient population.
VPM1002BC and the world-wide BCG shortage: Meanwhile, in 2012, Sanofi Pasteur halted production of their BCG for the treatment of bladder cancer, a measure which initiated the world wide BCG shortage, that – even 10 years later - has not been resolved. The shortage has led to significantly higher treatment costs, more cancer recurrences, and increased the rate of bladder removals in affected patients (Ourfali, Eur Urol Focus; PMID: 31005491). As such, new drugs are needed for the prevention of recurrence and progression of NMIBC. The FDA has facilitated the new drug development for NMIBC patients non-responsive to BCG therapy by allowing single arm trials for drug registration. This has led to the absurd situation that most of the new drugs for NMIBC are currently investigated in the BCG non-responsive setting while we are lacking sufficient amounts of BCG for the treatment of primary occurring NMIBC. Unfortunately, probably only an intellectual property-protected new BCG will provide enough incentive for a registration trial to solve the current shortage. VPM1002BC is such a potential new BCG and is manufactured utilizing state-of-the-art closed-loop processes and single-use equipment. This, combined with the fully scalable nature of fermentation in dedicated single-use bioreactors, ensures reliable and high-quality manufacturing that could meet the market demand to combat the global BCG shortages (Figure 2).


Figure 2. Top picture: Dedicated bulk manufacturing facility for VPM1002BC at Serum Institute of India Pvt. Ltd. Single-use equipment and closed-loop systems ensure sterility of the processes. Bioreactors (also single-use) are allow for high-volume, reliable manufacturing. Bottom picture: One of the largest isolator-based filling lines provides sterility assurance at the highest level and can fill and finish up to 150,000 vials in one batch.
Final remark: VPM1002BC has shown promising efficacy with a 3-yr recurrence-free survival rate of 43.7% in BCG pre-treated patients and very good tolerability and quality of live scores (Rentsch Eur Urol Onc; PMID: 35012889). We believe it is a candidate BCG that should further be developed, especially in order to overcome the BCG shortage that is harming our patients.
Proposed intracellular pathways induced by VPM1002: VPM1002 was created by replacing the urease C gene with the listeriolysin gene of Listeria monocytogenes. After its engulfment by antigen presenting cells, VPM1002 ends up in phagosomes. From there antigenic peptides are loaded on MHC II antigen presenting molecules (1). Lack of urease C allows acidification of the phagosome which promotes membrane perturbation by listeriolysin. Antigens from VPM1002 leak through perturbated membranes and then gain access to MHC I antigen presenting molecules in the cytosol (2). Membrane perturbation also leads to apoptosis which facilitates cross-priming (3). Double-stranded DNA egresses through the perturbated membrane of the phagosome and is sensed by absence in melanoma II (AIM II) which activates the inflammasome to generate bioactive IL-1β and IL-18 which cause inflammation (4). Double-stranded DNA also promotes formation of cyclic GMP–AMP synthase (cGAS) which is subsequently transformed into cyclic guanosine monophosphate adenosine monophosphate (cGAMP) which is sensed by the stimulator of IFN genes (STING) thereby promoting autophagy and type I IFN secretion (5). Autophagy facilitates immune stimulation and accelerated degradation of VPM1002. Listeriolysin comprises a PEST-like sequence composed of the amino acids prolin (P), glutamate (E), serine (S), threonine (T). This sequence causes rapid degradation of listeoriolysin in the cytosol by ubiquitination either directly or indirectly in autophagosomes (6). For reference, please refer to Front. Microbiol. 12:750124, 2021. © Fig. 1 is adapted from S.H.E. Kaufmann, Front. Microbiol. 12:750124, 2021, with permission from the author.
Written by: Cyrill A. Rentsch,1 Umesh Shaligram,2 Hitt Sharma,2 Manish Gupta,2 Gerald Parzmair3 and Stefan H. E. Kaufmann4
- Department of Urology, University Hospital Basel, Switzerland
- Serum Institute of India Pvt. Ltd., Pune, India
- Vakzine Projekt Management GmbH, Hannover, Germany
- Max Planck Institute for Infection Biology, Berlin, Germany
Read the Abstract


