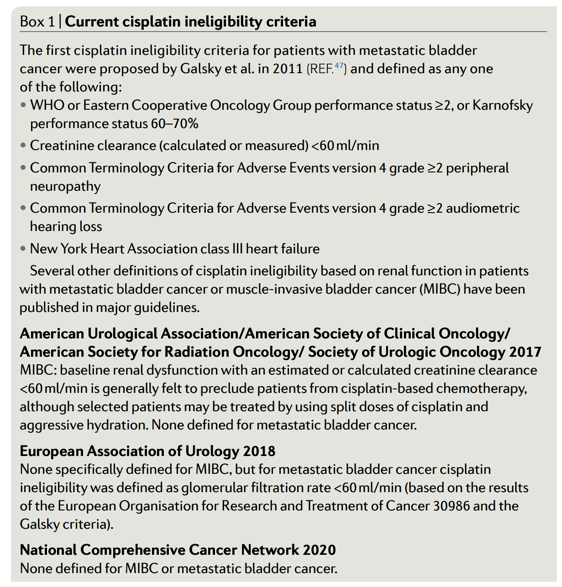
Current criteria for cisplatin ineligibility include an absolute creatinine clearance (CrCl) threshold of 60 mL/min as shown in Box 1. These criteria were however developed for patients with incurable, metastatic disease, for which the goal of treatment is palliative. When applied to the setting of MIBC, a renal function threshold of 60 mL/min excludes up to half of the patients from receiving NAC.6 This can lead to significant under-treatment and high systemic relapse rates. Therefore, for patients with MIBC where the treatment intent is curative, the use of the current cisplatin ineligibility criteria may not be appropriate.
Defining sufficient renal function for cisplatin-based NAC is challenging for a number of reasons.
- Measured CrCl through a timed urine collection is often limited by the inconsistent methods of sample collection, inconvenience, and cost.
- Calculated or estimated renal function using formulas such as the Cockcroft-Gault, Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI), or Modification of Diet in Renal Disease (MDRD) are often limited by accuracy. Notably, up to one-third of patients with MIBC will actually cross over the 60 ml/min threshold when a different formula is applied.7
- Among the three equations, the CKD-EPI may be optimal for determining cisplatin eligibility.
Accumulating data suggests that some patients with baseline renal impairment and a renal clearance of 40 – 60 ml/min can be safely treated with cisplatin. The risk of cisplatin-induced nephrotoxicity may have been over-estimated decades ago, and may actually be lower in the contemporary era. With better supportive care, cisplatin-induced nephropathy can also be partially or fully reversible in some patients. In the context of MIBC, some patients and clinicians might be willing to accept a slightly higher risk of cisplatin-induced nephrotoxicity for an increased chance of cure. Therefore, in the neoadjuvant setting, an absolute CrCl threshold of 60 mL/min should not be routinely used to exclude patients from NAC.
To better address these challenges in determining cisplatin eligibility for patients with MIBC, we have developed an algorithm, recognizing the need for a more patient-centered and multidisciplinary approach (Figure 1). We hope this will help to reduce under-treatment and harmonize clinic trial design, which may lead to improved overall outcomes in patients with MIBC.
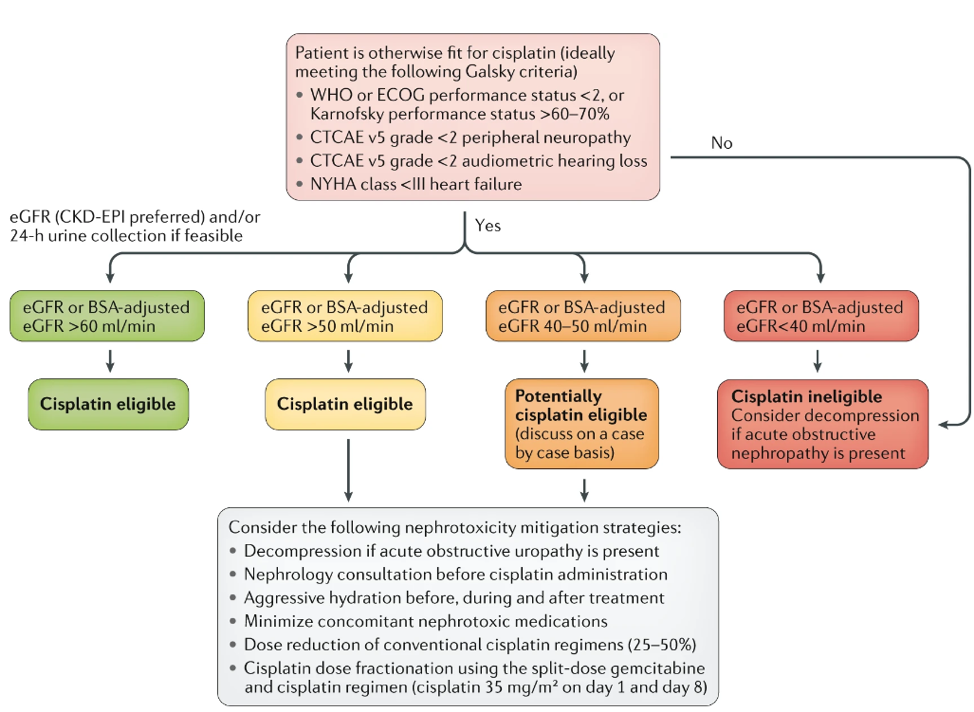
Figure 1. Proposed algorithm for determining eligibility for cisplatin-based NAC in patients with MIBC.
Written by: Di Maria Jiang, MD, Medical Oncologist, Princess Margaret Cancer Centre, Toroto, Canada; Srikala Sridhar, MD, MSc, FRCPC, Clinician Investigator, Cancer Clinical Research Unit (CCRU), Princess Margaret Cancer Centre, Toronto, Canda
References:
- Madersbacher S, Hochreiter W, Burkhard F, Thalmann GN, Danuser H, Markwalder R, Studer UE. Radical cystectomy for bladder cancer today - A homogeneous series without neoadjuvant therapy. J Clin Oncol. 2003; 21: 690–6. doi: 10.1200/JCO.2003.05.101.
- Tarin T V., Power NE, Ehdaie B, Sfakianos JP, Silberstein JL, Savage CJ, Sjoberg D, Dalbagni G, Bochner BH. Lymph node-positive bladder cancer treated with radical cystectomy and lymphadenectomy: Effect of the level of node positivity. Eur Urol. 2012; 61: 1025–30. doi: 10.1016/j.eururo.2012.01.049.
- Grossman HB, Natale RB, Tangen CM, Speights VO, Vogelzang NJ, Trump DL, deVere White RW, Sarosdy MF, Wood DP, Raghavan D, Crawford ED. Neoadjuvant Chemotherapy plus Cystectomy Compared with Cystectomy Alone for Locally Advanced Bladder Cancer. N Engl J Med [Internet]. 2003; 349: 859–66. Available from http://www.nejm.org/doi/full/10.1056/NEJMoa022148
- Advanced Bladder Cancer (ABC) Meta-Analysis Collaborators. Neoadjuvant Chemotherapy in Invasive Bladder Cancer : Update of a Systematic Review and Meta-Analysis of Individual Patient Data. European Urology. 2005. p. 202–6. doi: 10.1016/j.eururo.2005.04.006.
- Petrelli F, Coinu A, Cabiddu M, Ghilardi M, Vavassori I, Barni S. Correlation of pathologic complete response with survival after neoadjuvant chemotherapy in bladder cancer treated with cystectomy: A meta-analysis. Eur Urol [Internet]. European Association of Urology; 2014; 65: 350–7. doi: 10.1016/j.eururo.2013.06.049.
- Iff S, Craig JC, Turner R, Chapman JR, Wang JJ, Mitchell P, Wong G. Reduced Estimated GFR and Cancer Mortality. Am J Kidney Dis [Internet]. Elsevier Inc; 2014; 63: 23–30. doi: 10.1053/j.ajkd.2013.07.008.
- Dash A, Galsky MD, Vickers AJ, Serio AM, Koppie TM, Dalbagni G, Bochner BH. Impact of Renal Impairment on Eligibility for Adjuvant Cisplatin-Based Chemotherapy in Patients With Urothelial Carcinoma of the Bladder. Cancer. 2006; 107: 506–13. doi: 10.1002/cncr.22031.
Read the Abstract


