Washington, DC (UroToday.com) As part of the SUO 2019 advanced prostate cancer session, Dr. Samuel Denmeade discussed his work with bipolar androgen therapy (BAT) for men with castration resistant prostate cancer (CRPC). Dr. Denmeade reminds us that metastatic prostate cancer remains an incurable disease, with a median overall survival in the CRPC state of three years. The mainstay of treatment is androgen deprivation therapy (ADT), however it is associated with many side effects:
- Depression
- Decreased muscle mass
- Breast enlargement
- No libido/sexual impotence
- Decreased bone mass
- Abdominal fat
- Fatigue
- Lack of focus
One of the main challenges of treating advanced prostate cancer is the development of resistance with each subsequent line of therapy. For example, enzalutamide in PREVAIL had a PSA progression-free survival of only 11.2 months. According to Dr. Denmeade, there are three phases of androgen inhibition: shock, adaptation, and resistance. The shock phase is when there is androgen receptor activity inhibition, which is followed by adaptation, namely androgen receptor overexpression, gene amplification, and development of ligand-independent androgen receptor variants.
The hypothesis for BAT is that men with CRPC could respond to rapid cycling between polar extremes of supraphysiologic and castrate testosterone levels, whereby rapid cycling disrupts adaptive autoregulation of the androgen receptor. Adaptive downregulation of the androgen receptor expression may re-sensitize CRPC to androgen ablative therapies. Schematically, BAT is as follows:
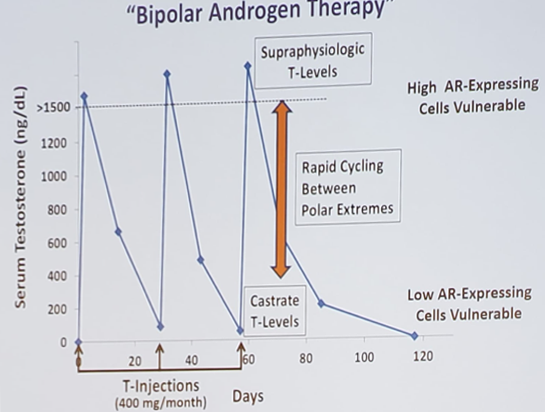
Dr. Denmeade’s group conducted the RE-sensitizing with Supraphysiologic Testosterone to Overcome Resistance (The RESTORE Study), which the schema is as follows:
In the post-enzalutamide patients, the >50% PSA response was 30%, any PSA decline was 51%, the median progression free-survival was 8.6 months, and the objective response rate after 3 cycles of BAT was 43%.1 Additionally, 71% of patients with a >50% decrease in PSA had a median duration response of 4.8 months.
Dr. Denmeade’s group subsequently designed the TRANSFORMER phase II trial:
For the initial response results, progression free survival and objective response rate were comparable between the BAT and enzalutamide arms were comparable, however the time to PSA progression favored the enzalutamide arm (3.8 vs 2.8 months; p=0.012). For the crossover response results, objective response favored enzalutamide (28.6% vs 8.1%, p=0.045), as did time to PSA progression (10.9 vs 1.2 months, p=0.0005). Finally, BAT + enzalutamide sequencing increases overall progression free survival (PFS2):
Dr. Denmeade concluded with several take home points:
- Pharmacologic testosterone (BAT) can be given safely to asymptomatic men with castration-resistant prostate cancer
- Objective response and PSA response were observed in some men
- BAT may re-sensitize CRPC to androgen ablative therapies
- BAT improves quality of life in some men
Presented by: Samuel Denmeade, Co-Director Prostate Cancer Program, Professor of Oncology, Johns Hopkins Hospital, Baltimore, Maryland
Written by: Zachary Klaassen, MD, MSc – Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, Twitter: @zklaassen_md at the 20th Annual Meeting of the Society of Urologic Oncology (SUO), December 4 - 6, 2019, Washington, DC
References:


