In this study, the authors look at a novel immunomodulatory approach. The infused autologous dendritic cells transduced by a replication deficient adenovirus comprised of GM-CSF+CAIX. The authors generated a fusion gene construct of GM-CSF and CAIX transduced into autologous dendritic cells (DCs). CAIX is a known marker of renal cell carcinoma, providing the specificity required for therapy. This was a phase 1 open-label dose-escalation study aiming to primarily assess safety and dosing. Nine patients in three dose-escalation cohorts (5,15, and 50 X 106 cells/administration) were injected based on a 3+3 design. This amounted to a total of 9 patients, 3 in each arm.
From a methodology standpoint, an enzyme-linked immunospot (ELISpot) assay determined the frequency of CAIX-specific IFN-γ producing T-cells in a patient’s blood sample as baseline. 15-mer overlapping peptides from CAIX-protein, AdV5-pepton, and controls (+/-) were plated in Elispot plates pre-coated with anti-IFN-γ antibody. Subsequent to assay development, the number of T-cells responding to CAIX was recorded as the lower limit of detection (LLD) – determined by 7 spots. After subtracting the backgrounds, fold-change was calculated with respect baseline. Positive immunological response was defined as the mean fold-change plus two.
Further IHC assessment was obtained from two patients - patients #4 (with progressive disease) and #8 (with stable disease). IHC for CAIX, CD4/8, Ki67, GrZ8, PD1/L1 were assessed. The samples were scored based on percent positivity and staining intensity. Tissue was obtained from the primary tumor prior to vaccination, and the target tumor at the end of the study period (18 months).
ELISpot showed consistently positive responses against CAIX upon vaccination with DC-vaccine, more prominently in patients in cohort 3 (high dose) and in those with longer time to progression. However, none of the treated patients showed an objective response.
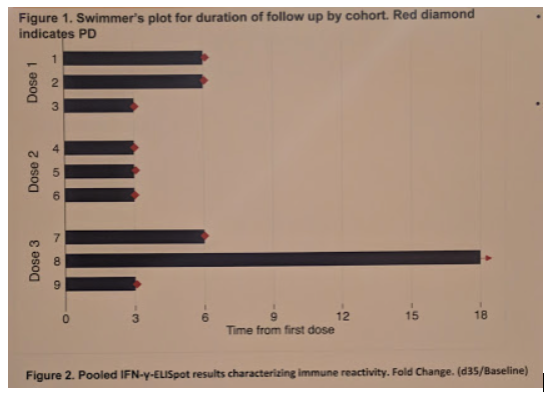
Patient #8, however, achieved stable disease (SD) lasting 18 months. This patient had more than 2-fold change in immune response over baseline on day 35 and 60 after the first vaccination cycle.
All nine patients showed different degrees of immunological reaction to AdV5 at baseline and elevation at the end of the study.
IHC showed that both patient #4 and #8 patients had high CAIX expression in primary tumor and on the target lesion post vaccination. Immune infiltrates were seen at baseline in both subjects, with predominant CD4/8 T-cells in patient #8 with a high PD-1 expression in infiltrating lymphocytes without PD-L1 expression in the tumor environment. Full results seen below:

Based on this, the authors conclude that vaccination with DC-AdGMCAIXv may elicit robust immunologic response against CAIX in patients with ccRCC. However, the lack of significant objective response is disconcerting, and further studies are required. The findings of high PD-1 expression in the patient with stable disease in both the primary tumor and target lesion warrants future efforts to explore how combination therapies with biological response modifiers may further enhance clinical responses.
This study provides biologic rationale for further evaluation, but obviously the degree of response is concerning.
Speaker: Izak Faiena, MD
CO-AUTHORS: Nazy Zomorodian; Beata Berent-Maoz; Ankush Sachadeva; Adrian Bot; Fairouz Kabinnovar; Jonathan Said; Gardenia Cheung-Lau; Jia Pang; Mignonette Macaballi; Thinle Chodon; Xiaoyan Wang; Paula Cabrera; Paula Kaplan-Lezco; Sandy Liu; Begonya Comin-Anduix; Allan Pantuck; Arie Belldegrun; Karim Chamie and Alexandra Drakaki
Written by: Thenappan Chandrasekar, MD, Clinical Instructor, Thomas Jefferson University, twitter: @tchandra_uromd, @TjuUrology, at the 19th Annual Meeting of the Society of Urologic Oncology (SUO), November 28-30, 2018 – Phoenix, Arizona


