For the treatment of radium Ra 223 dichloride (Xofigo®) the company provides a great single source of support for ordering a treatment, patient insurance, and benefits verification, reimbursement support, and patient assistance.
For this specific treatment patients are required to copay 0$ (if they meet the eligibility criteria), and the company also provides travel assistance to and from the treatment site. As for other treatments, it is important to confirm the state requirements for radiation medical program license (RML).
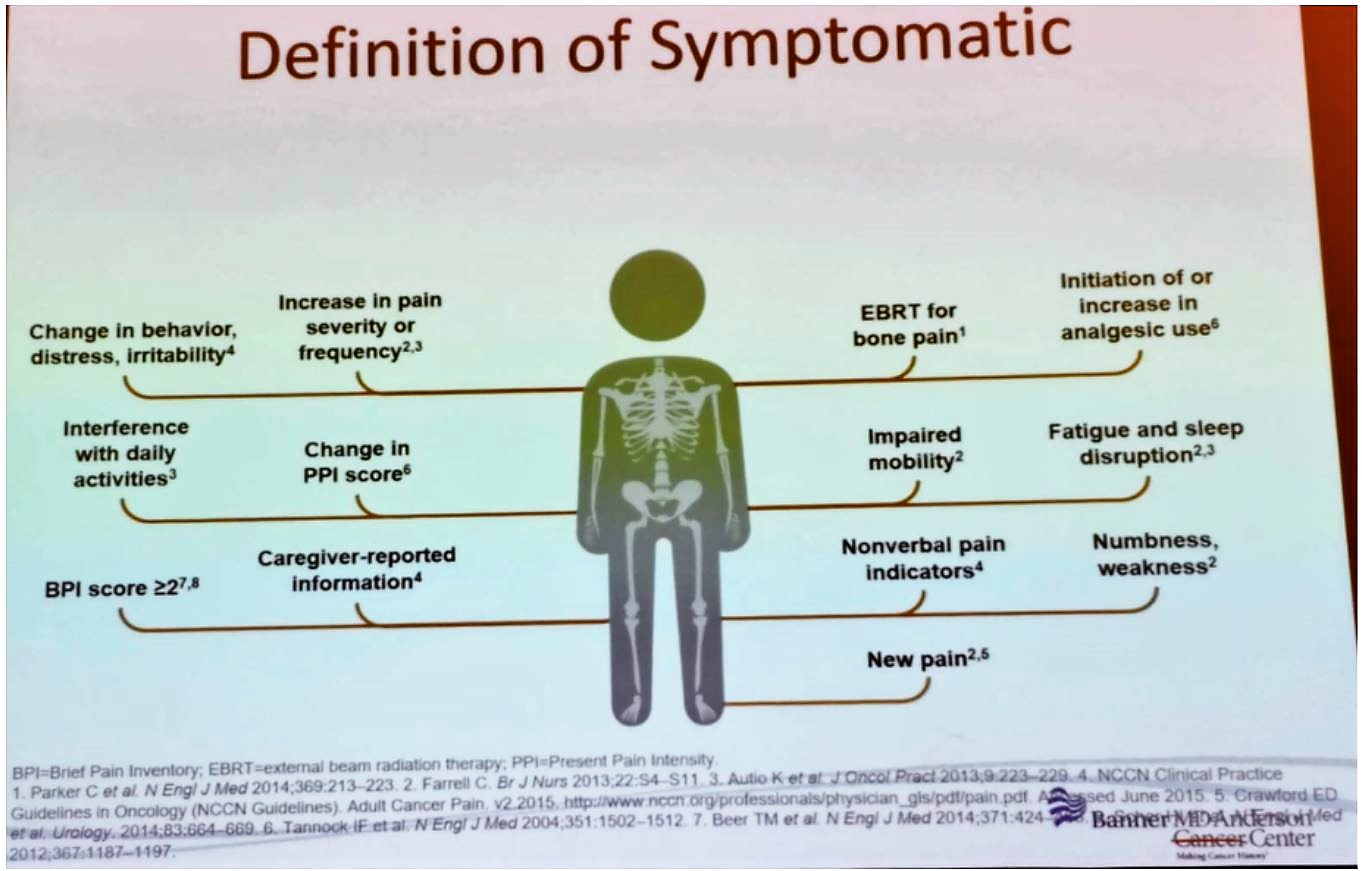
Once the clinic has been set up and treatment is available, identifying the appropriate patients is required. For this specific treatment with Radium-223, the right patients are those with castration-resistant prostate cancer, defined as progressive disease despite castrate levels of testosterone. This treatment has been shown to be beneficial in this patient population in the ALSYMPCA trial.1 The patient does not have to be in severe pain but is required to have symptomatic bone metastases (Figure 1) with no evidence of visceral metastatic disease.
Figure 1 – Definition of symptomatic disease:
One week before the first dose, the patient is required to have:
- An absolute Neutrophil count > 1.5 *109/L
- Platelets >=100 *109/L
- Hemoglobin >=10 gr/dl
Dr. Koo concluded his talk discussing the follow-up of these patients and discussing treatment discontinuation. According to the advanced prostate cancer consensus published in 2015 assessing various questions regarding the treatment with Radium-223, showing that most physicians thought that PSA, total alkaline phosphatase, LDH, and PSA need to be assessed every 3-6 months.2 Treatment should be discontinued If hematologic values drop and do not recover within 6 to 8 weeks after the last administration, despite receiving supportive care. If there is a significant progression of the disease with symptoms or development of visceral metastatic disease, treatment should be stopped as well.
Presented by: Phillip Koo, MD, Division Chief of Diagnostic Imaging at the Banner MD Anderson Cancer Center in Arizona
Written by: Hanan Goldberg, MD, Urology Department, SUNY Upstate Medical University, Syracuse, New York, USA, Twitter: @GoldbergHanan at the 2019 SNMMI Therapeutics Conference: Therapies, Theranostics, and Building Your Radionuclide Clinical Practice, October 25-27, 2019 in Las Vegas, Nevada
References:
1. Parker C, Nilsson S, Heinrich D, et al. Alpha Emitter Radium-223 and Survival in Metastatic Prostate Cancer. New England Journal of Medicine 2013; 369(3): 213-23.
2. Gillessen S, Omlin A, Attard G, et al. Management of patients with advanced prostate cancer: recommendations of the St Gallen Advanced Prostate Cancer Consensus Conference (APCCC) 2015. Annals of oncology : official journal of the European Society for Medical Oncology 2015; 26(8): 1589-604.


