Athens, Greece (Urotoday.com) Neoadjuvant chemotherapy (NAC) in bladder cancer has been proven to improve overall survival in patients with muscle-invasive bladder cancer (MIBC) over 15 years ago1. Accurate preoperative prediction of pathological T0 could perhaps spare cystectomy in a significant portion of patients2. NAC compared to TURBT alone has shown a significant advantage with a higher percentage of patients reaching pT0 (30% vs. 12%). Patients reaching Pt0 following NAC have significantly better survival curves than those not reaching Pt0, perhaps enabling them to keep their bladder and not undergo radical cystectomy.
However, the absence of tumor on repeat transurethral resection of bladder tumor does not predict final pathological T0 stage in bladder cancer patients treated with radical cystectomy, i.e. clinical T0 does not equal pathological T03.
Next, Dr. Stratton discussed whether improvements in clinical evaluation provide a better assessment of response to NAC. In the AUA meeting in 2018 a study was presented where the authors used cystoscopy to systematically evaluate the bladder prior to radical cystectomy and performed targeted and systematic biopsies and then compared the results to the pathology of the final specimen derived from radical cystectomy4. A total of 35 patients underwent radical cystectomy with 60% of them receiving NAC. The systematic evaluation with cystoscopy and biopsy before radical cystectomy predicted pT0 in 18 patients, but the actual pT0 was found in only 11 patients, showing negative predictive value (NPV) of 55.6%.
Another question raised by Dr. Stratton was whether chemotherapy response biomarkers improve the identification of patients with clinical T0 disease following NAC. A study evaluating DNA repair genes predicted response to NAC in muscle-invasive bladder cancer. The response to NAC in this study compared to gene alterations, with DNA-repair associated genes appearing to predict response and clinical benefit after NAC5.
There is a phase 2 trial (RETAIN BLADDER) which includes risk enabled therapy after initiating NAC (Figure 2). The primary endpoint is metastases free survival (MFS) at 2 years. In this trial, following TURBT and NAC, patients who have cT0 will undergo active surveillance.
Figure 2 – RETAIN BLADDER trial design: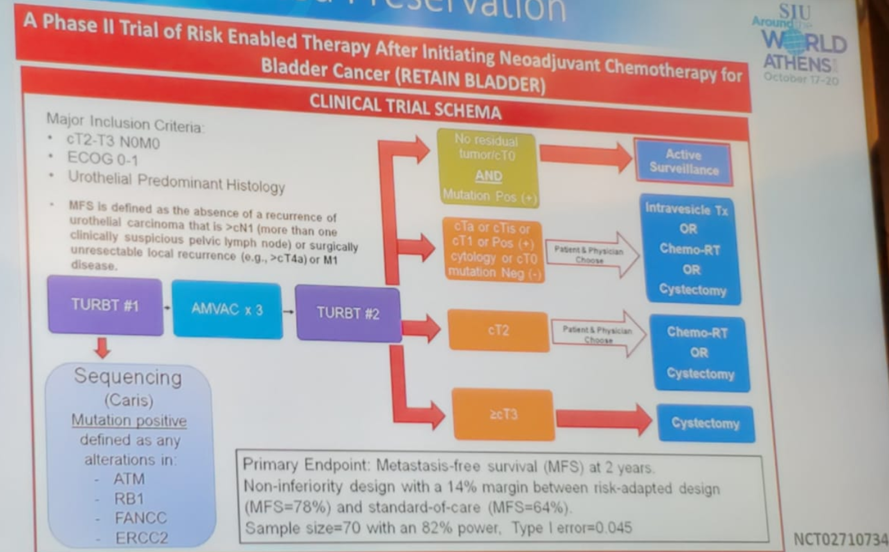
Another ongoing phase 2 trial is the A031701 which is a phase II study of dose-dense gemcitabine and cisplatin in patients with MIBC with bladder preservation for those whose tumors harbor deleterious DNA damage response (DDR) gene alterations (Figure 3).
Figure 3 – A031701 trial design: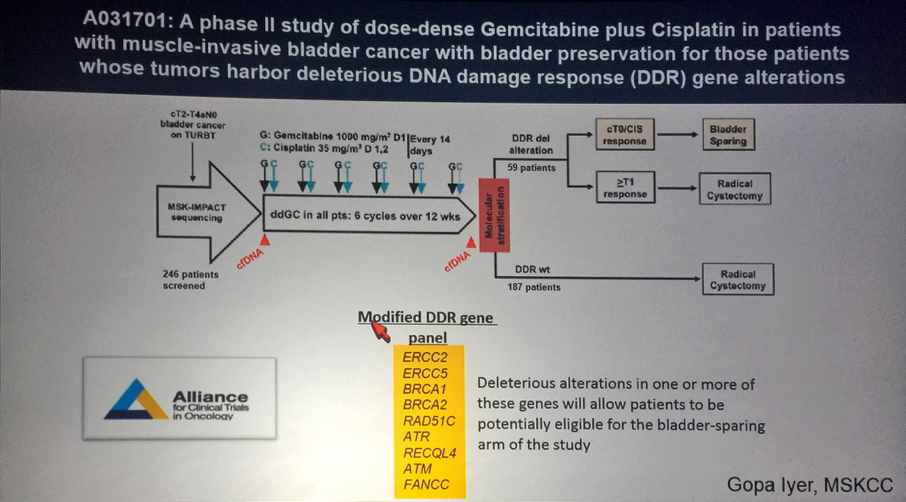
In conclusion, NAC and quality surgery work synergistically. Data show that bladder preservation may be an option for select patients. Importantly, a complete response does not equal cure, and clinical T0 does not equal pathological T0. Lastly, biomarker-based approaches to bladder preservation may overcome the current challenges of patient selection.
References:
- Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant Chemotherapy plus Cystectomy Compared with Cystectomy Alone for Locally Advanced Bladder Cancer. New England Journal of Medicine 2003; 349(9): 859-66.
- Lavery HJ, Stensland KD, Niegisch G, Albers P, Droller MJ. Pathological T0 Following Radical Cystectomy with or without Neoadjuvant Chemotherapy: A Useful Surrogate. Journal of Urology 2014; 191(4): 898-906.
- Kukreja JB, Porten S, Golla V, et al. Absence of Tumor on Repeat Transurethral Resection of Bladder Tumor Does Not Predict Final Pathologic T0 Stage in Bladder Cancer Treated with Radical Cystectomy. European Urology Focus 2018; 4(5): 720-4.
- Parker D, Asghar A, O'Neill J, et al. LBA5 INITIAL RESULTS OF A PROSPECTIVE COHORT STUDY EVALUATING RELIABILITY OF ENDOSCOPIC EVALUATION IN PREDICTING PT0 DISEASE AT THE TIME OF RADICAL CYSTECTOMY: WHERE AND HOW DOES CYSTOSCOPY FALL SHORT? Journal of Urology 2018; 199(4S): e578-e9.
- Plimack ER, Dunbrack RL, Brennan TA, et al. Defects in DNA Repair Genes Predict Response to Neoadjuvant Cisplatin-based Chemotherapy in Muscle-invasive Bladder Cancer. Eur Urol 2015; 68(6): 959-67.
Written by: Hanan Goldberg, MD, Urology Department, SUNY Upstate Medical University, Syracuse, New York, USA, Twitter: @GoldbergHanan at the 39th Congress of the Société Internationale d'Urologie, SIU 2019, #SIUWorld #SIU2019, October 17-20, 2019, Athens, Greece


