To assess the clinical role for this combination approach, the authors enrolled cisplatin-ineligible patients who had not previously received systemic therapy for la/mUC.
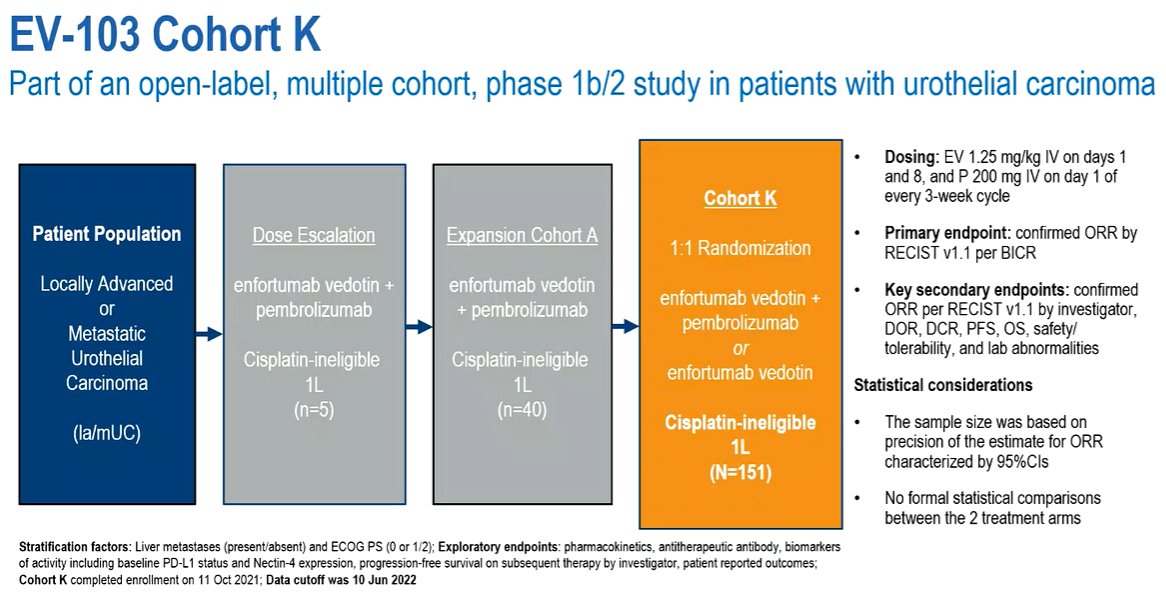
Once enrolled, patients were randomized 1:1 to EV (1.25 mg/kg) as monotherapy on Days 1 and 8 or in combination with pembrolizumab (200 mg) on Day 1 of 3-week cycles (EV-103, NCT03288545). The primary endpoint of this study was confirmed objective response rate (ORR) per RECIST v1.1 by BICR with secondary endpoints including duration of response (DOR) and safety (treatment-related adverse events, TRAEs). There were no formal statistical comparisons between treatment arms.
The authors enrolled 149 patients, of whom 76 were treated with the combination of EV and pembrolizumab, and the remaining 73 received EV alone. Notably, renal impairment was the primary reason patients were deemed cisplatin-ineligible (63% of those in the EV+pembro and 60% of those in the EV monotherapy arms).
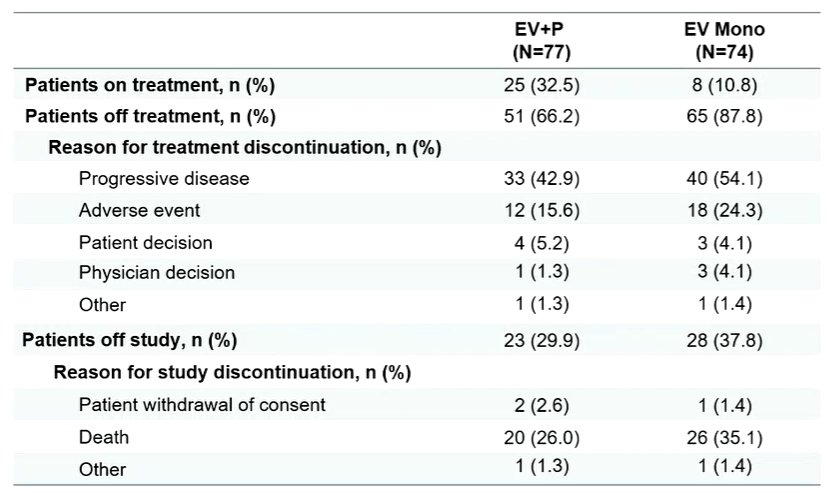
Disease progression was the primary reason to stop therapy in both arms.
The confirmed ORR was 64.5% (95%CI 52.7 to 75.1) among patients treated with the combination of EV and pembrolizumab, with a median DOR not reached. Among those treated with EV monotherapy, the confirmed ORR was 45.2% (95% CI 33.5 TO 57.3) and median DOR was 13.2 months (95% CI 6.1 to16.0).

Further, 97.1% of patients treated with EV plus pembrolizumab had a reduction in tumor volume. To date, survival data are somewhat immature. However, there appears to be better median progression-free survival among those receiving the combination approach, compared to EV monotherapy, though overall survival did not differ.

Importantly, activity was seen independent of Nectin4 expression.
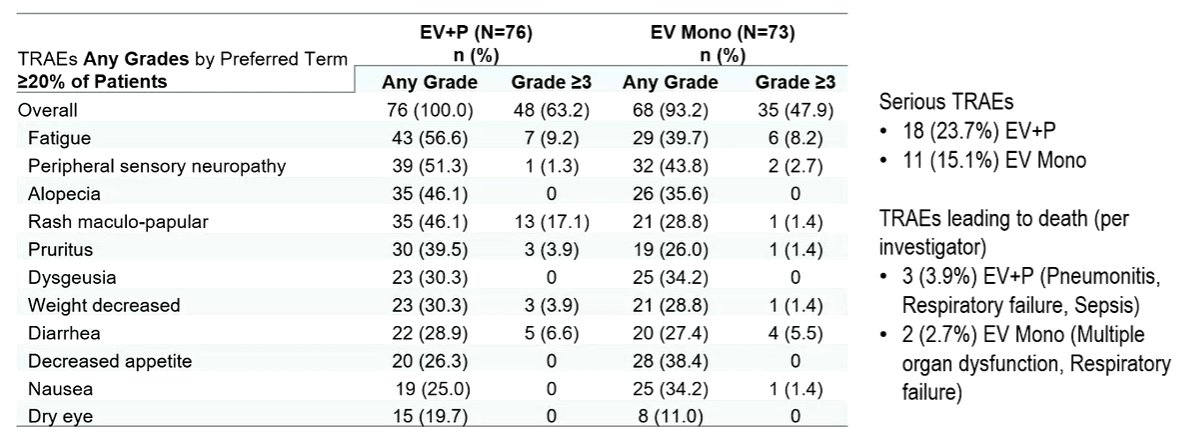
TRAEs of special interest were somewhat more common in the combination group and included skin reactions (EV+pembrolizumab, n=51 [67.1%]; EV, n=33 [45.2%]), peripheral neuropathy (EV+ pembrolizumab, n=46 [60.5%]; EV, n=40 [54.8%]), ocular disorders (eg, dry eye and blurred vision; EV+ pembrolizumab, n=20 [26.3%]; EV, n=21 [28.8%]), and hyperglycemia (EV+ pembrolizumab, n=11 [14.5%]; EV, n=8 [11.0%]). The majority of treatment-related AEs were Grade ≤2.
Thus, Dr. Rosenberg concluded that the combination of EV and pembrolizumab showed high ORR with rapid responses and median DOR not reached in a first-line cisplatin-ineligible population of patients with la/mUC. The safety profile was tolerable and generally consistent with the known profile for EV+pembrolizumab. EV monotherapy was consistent with prior experience. There are currently three ongoing phase 3 trials assessing the combination of EV and pembrolizumab.
Presented by: Jonathan E. Rosenberg, MD, Chief of the Genitourinary Medical Oncology Service, Division of Solid Tumor Oncology; and the Enno W. Ercklentz Chair at Memorial Sloan Kettering Cancer Center, New York City, New YorkWritten by: Christopher J.D. Wallis, University of Toronto Twitter: @WallisCJD during the 2022 European Society for Medical Oncology (ESMO) Annual Congress, 9-13 September 2022.


