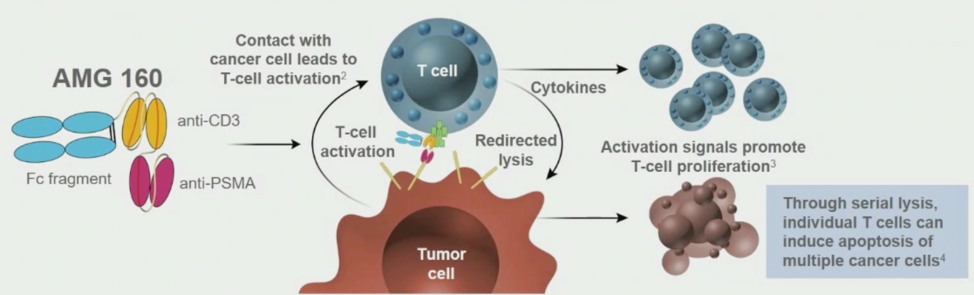
At the prostate cancer session during the virtual ESMO 2020 annual meeting, Dr. Ben Tran and colleagues presented their preliminary results from the dose exploration portion of an ongoing phase I study of AMG 160 in mCRPC (NCT03792841).
For this study, eligible patients had mCRPC refractory to prior novel hormonal therapy and 1–2 taxane regimens, as well as evidence of progressive disease. Key exclusion criteria included active autoimmune disease or requiring immunosuppressive therapy, prior PSMA-targeted therapy (however, patients treated with PSMA radionuclide therapy were eligible), as well as patients with CNS metastases, leptomeningeal disease, or spinal cord compression. AMG 160 was administered as a short IV infusion every two weeks at doses of 0.003–0.9 mg. The combination of AMG 160 plus pembrolizumab was also evaluated (results not presented). The primary objectives of this study were to evaluate safety and tolerability, as well as the maximum, tolerated dose, and recommended phase II dose. Secondary objectives were to characterize the pharmacokinetics and to evaluate preliminary antitumor activity. Finally, exploratory objectives were to evaluate biomarkers of activity and to identify potential patient selection biomarkers. A summary of the trial design is as follows:
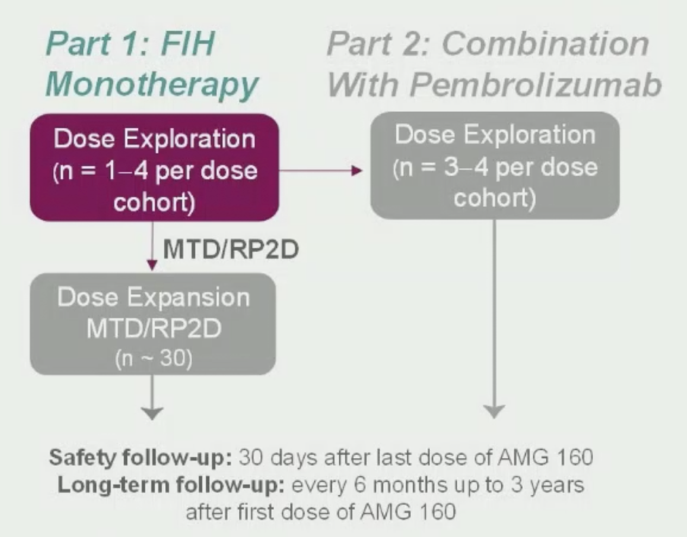
Cytokine release syndrome, measured as an adverse event, was graded according to the Lee criteria:1
As of the data cutoff of July 20, 2020, 43 patients had received ≥ 1 dose of AMG 160 monotherapy at 6 dose levels, and 19 patients (44.2%) were still on treatment (6 for ≥ 6 months). The median age was 66.0 (range: 49–78) years, the majority of patients were Caucasian (79.1%), and 26 patients (60.5%) had received ≥ 4 prior lines of therapy. The median number of prior lines of therapy was 4 (range: 1-9), and the median PSA at baseline was 79.2 (range 0.1-4035) ng/dL; 34.9% of patients had RECIST-measurable disease. Among the 43 patients, 41 (95.3%) experienced a treatment-related adverse event, most commonly cytokine release syndrome (n=30, 90.7% all grade; n=11, 25.6% grade 3). Cytokine release syndrome was reversible, manageable, and most severe in cycle 1, with associated fever, hypotension, transient transaminitis, nausea/vomiting, and/or diarrhea. There were 26 patients (60.5%) that had grade 2 cytokine release syndrome as the worst grade, and 11 patients (25.6%) that had grade 3 cytokine release syndrome as the worst grade; 4 patients (9.3%) experienced reversible atrial fibrillation in the setting of cytokine release syndrome/tachycardia. Prophylactic mitigations to improve the cytokine release syndrome profile in the cycle 1 priming cohort included:
- Dose priming: lower run-in dose before maintenance target dose
- Dexamethasone premedication: 8mg PO and 8mg IV before AMG 160 dose
- Prophylactic IV hydration: 1L normal saline after the AMG 160 dose
There were no grade 5 events and none of the treatment-related adverse events resulted in treatment discontinuation. Six of 30 patients (20.0%) assessed developed antidrug antibodies affecting drug exposure between cycles 1 and 10, however, no adverse events associated with antidrug antibodies were observed.
At the data cutoff, the maximum tolerated dose had not been reached. AMG 160 demonstrated efficacy with long-term responses. There was a confirmed PSA response in 27.6% of patients, an unconfirmed PSA response in 11.4% of patients, and a CTC0 response in 23.1% of patients. Among patients with RECIST measureable disease, 13.3% had confirmed partial response, 6.7% had unconfirmed partial response, and 53.3% had stable disease. A summary of efficacy is as follows:
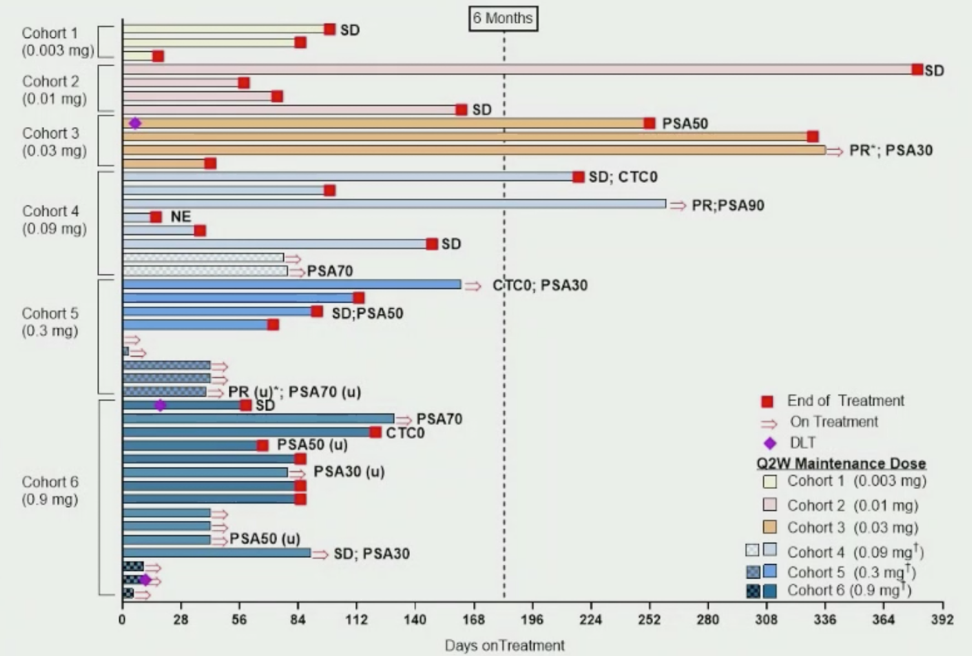
PSA reductions (best response) were dose-dependent and occurred in 24/35 (68.6%) of evaluable patients at the data cutoff of July 20, 2020, whereas PSA reductions >50% occurred in 12/35 (34/3%) of evaluable patients:
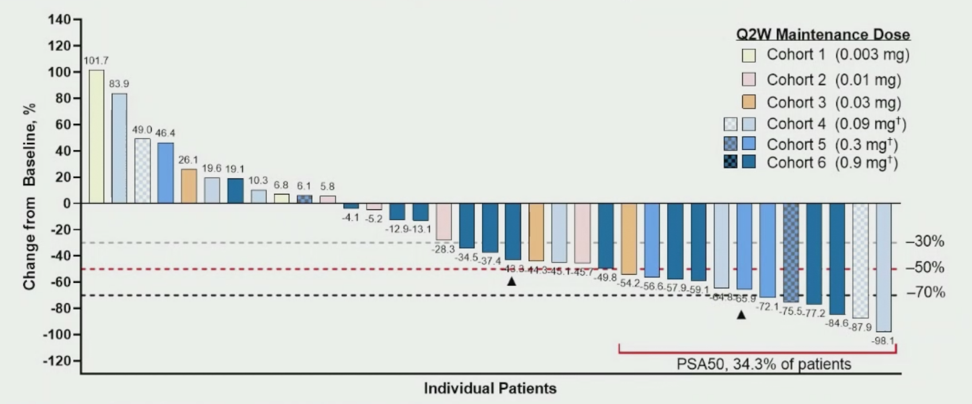
Dr. Tran then provided three patient examples of particularly deep responses to AMG 160:
- Patient 1: had prior surgery, radiotherapy, and four systemic therapies (docetaxel, enzalutamide, bicalutamide, and talazoparib) – treated with 0.09 mg AMG 160 with cycle 1 priming who had a rapid PSA drop in 1 month with a durable PSA response
- Patient 2: had prior surgery and three systemic therapies (docetaxel, enzalutamide, and sipuleucel-T) – treated with 0.09 mg AMG 160 with cycle 1 priming who had a rapid PSA drop in 1 month with a durable PSA response
- Patient 3: had prior radiotherapy and four systemic therapies (apalutamide, docetaxel, sipuleucel-T, and radium-223) – treated with 0.3 mg AMG 160 with cycle 1 priming who had a rapid PSA drop in 1 month (limited longer-term follow-up)
Dr. Tran concluded his presentation of the phase I AMG 160 trial with the following take-home messages:
- AMG 160 had a manageable safety profile as monotherapy: cytokine release syndrome was reversible and manageable with priming doses and standard mitigations, and there were no grade 5 treatment-related adverse events or treatment discontinuations
- In heavily pre-treated patients, AMG 160 showed preliminary evidence of efficacy: 68.6% of patients showed any PSA decline across all monotherapy dose cohorts, 34.3% had a PSA reduction >50%, and among 15 patients with measurable disease, there were 3 partial responses and 8 patients with stable disease
- 44.2% of patients remained on AMG 160 at the time of data analysis, with 6 (14.0%) of patients continuing treatment for more than 6 months
- The maximum tolerated dose had not been reached and dosing optimization of AMG 160 continues as the study nears the recommended phase II dose
- Investigation of AMG 160 in combination with pembrolizumab is in progress
Presented by: Ben Tran, MBBS, FRACP, Medical Oncology Department, Peter MacCallum Cancer Centre, Melbourne, Australia
Co-Authors: L. Horvath,2 T. Dorff,3 M. Rettig,4 M.P. Lolkema,5 J-P. Machiels,6 S. Rottey,7 K. Autio,8 R. Greil,9 N. Adra,10 C. Lemech,11 M. Minocha,12 F-C. Cheng,13 H. Kouros-Mehr,14 K. Fizazi15
Affiliations: 2 Medical Oncology, Chris O'Brien Lifehouse, Camperdown, Australia, 3 Internal Medicine, City of Hope, Duarte, CA, USA, 4 Medical Oncology, UCLA, Los Angeles, CA, USA, 5 Medical Oncology, Erasmus University Medical Center, Rotterdam, Netherlands, 6 Oncology, Cliniques Universitaires St. Luc, Brussels, Belgium, 7 Drug Research Unit Ghent, Ghent University, Ghent, Belgium, 8 Medical Oncology, Memorial Sloan Kettering Cancer Center, New York, NY, USA, 9 3rd Medical Department, Paracelsus Medical University Salzburg, Salzburg Cancer Research Institute-CCCIT and Cancer Cluster, Salzburg, Austria, 10 Urology, Indiana University School of Medicine, Indianapolis, IN, USA, 11 Medical Director, Scientia Clinical Research, Randwick, Australia, 12 Clinical Pharmacology Modeling + Simulation, Amgen Inc., Thousand Oaks, CA, USA, 13 Biostatistical Sciences, Amgen Inc., Thousand Oaks, CA, USA, 14 Translational Medicine, Amgen Inc., Thousand Oaks, CA, USA 15 Cancer Medicine Department, Institut Gustave Roussy, Villejuif, France
Written by: Zachary Klaassen, MD, MSc – Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia Twitter: @zklaassen_md at the 2020 European Society for Medical Oncology Virtual Congress (#ESMO20), September 19th-September 21st, 2020.
References:
- Lee DW, Gardner R, Porter DL, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014 Jul 10;124(2):188-195.
Related Content:
ESMO Virtual Congress 2020: Novel Immunotherapy for Prostate Cancer - AMG 160 - PSMA-Targeted, Bispecific T-Cell Engager (BiTE®) Immune Therapy for Metastatic Castration-Resistant Prostate Cancer


