Dr. Powles began by highlighting the DANUBE trial design. This trial enrolled patients with untreated unresectable or metastatic urothelial carcinoma and randomized them in a 1:1:1 fashion to durvalumab monotherapy, durvalumab, and tremelimumab, or standard of care chemotherapy. The authors examined a number of outcomes including overall survival, progression-free survival, objective response rate, and duration of response including both the intention to treat population and PD-L1 high population and examining comparisons of durvalumab monotherapy compared to chemotherapy and of durvalumab plus tremelimumab compared to chemotherapy.
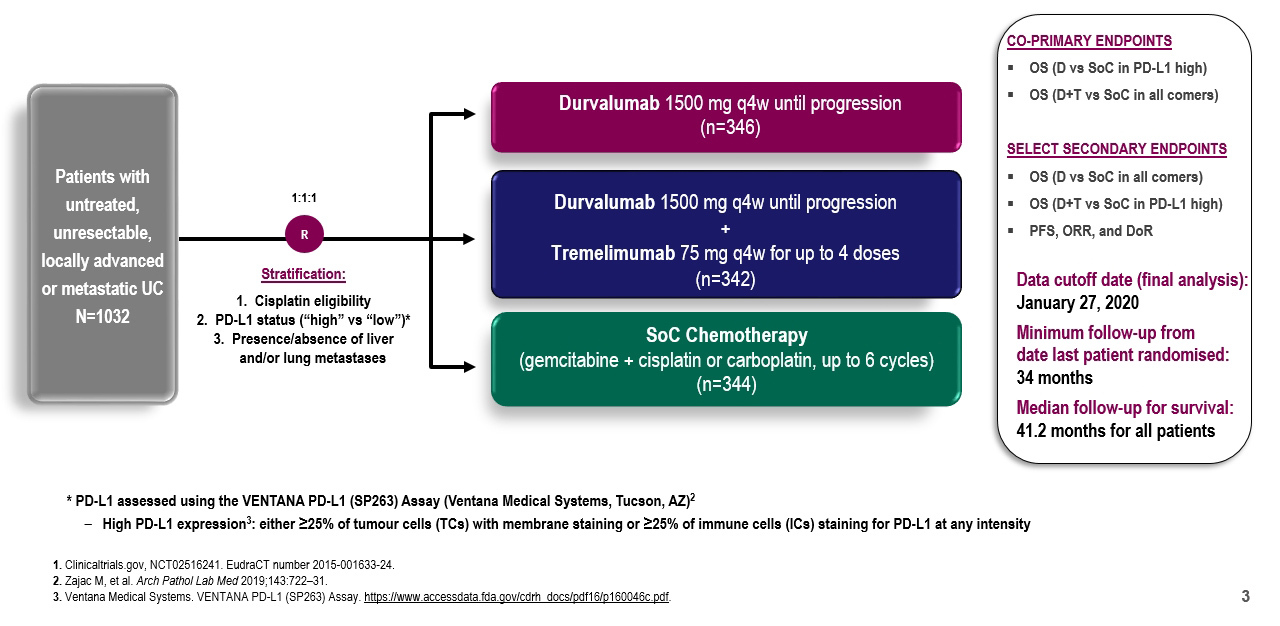
As expected in a large, phase III trial, the patient populations were well balanced between the three groups with respect to baseline characteristics including age, sex, region, histology, performance status, site of the primary tumor, liver/lung metastasis, lymph node only metastases, PD-L1 expression, cisplatin eligibility, and Bajorin risk factors. Notably, roughly 60% of patients were PD-L1 positive.
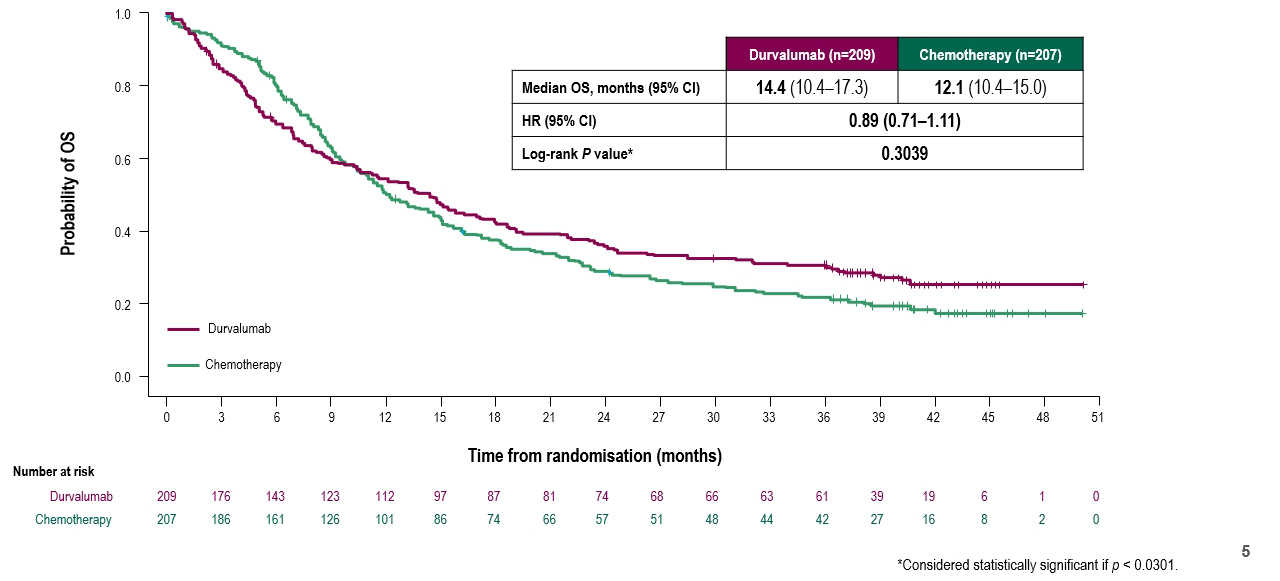
Assessing the first of the co-primary endpoints, Dr. Powles highlighted that there was no statistically significant difference in overall survival compared to chemotherapy in the PD-L1 high population, over a median follow-up of 41 months. He noted that the non-proportional curves indicated that chemotherapy outperformed durvalumab in the early period (~9 months) while the converse was true moving forward.
Subgroup analyses, as presented in forest plots, failed to demonstrate any evidence of effect modification to suggest subgroups in whom an immunotherapy approach would be particularly useful (or detrimental).
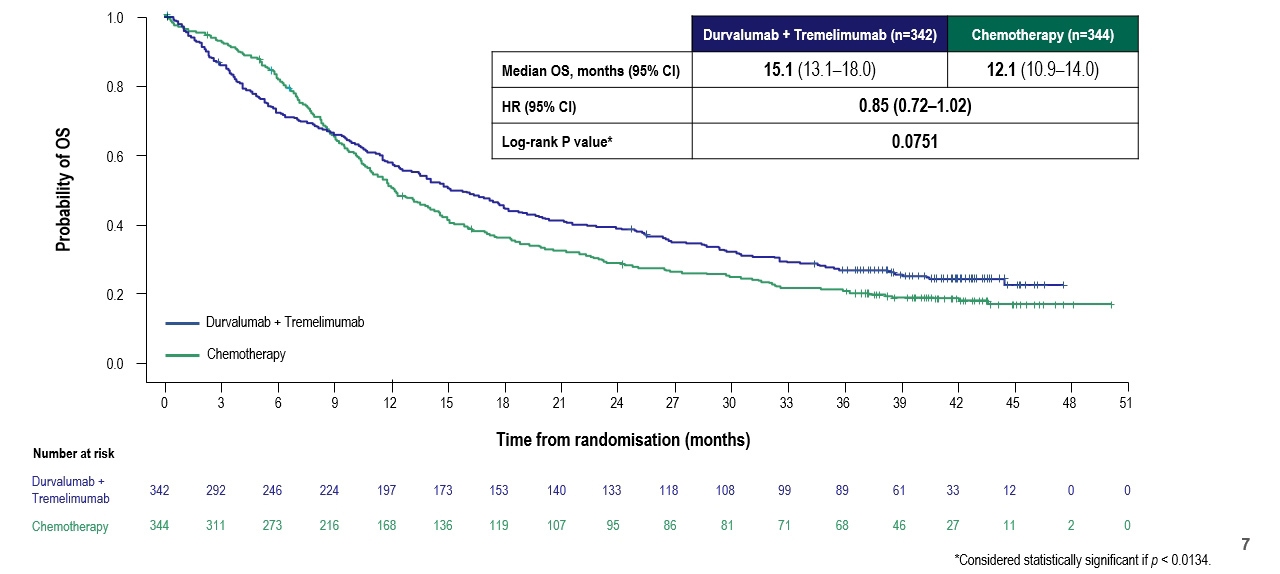
Dr. Powles then discussed the second co-primary endpoint, of overall survival in the comparison of durvalumab and tremelimumab versus chemotherapy in the intent to treat (ITT) population. Results were very similar to the other primary outcome with an early benefit to chemotherapy followed by an apparent benefit to the immunotherapy doublet, without statistically significant differences.
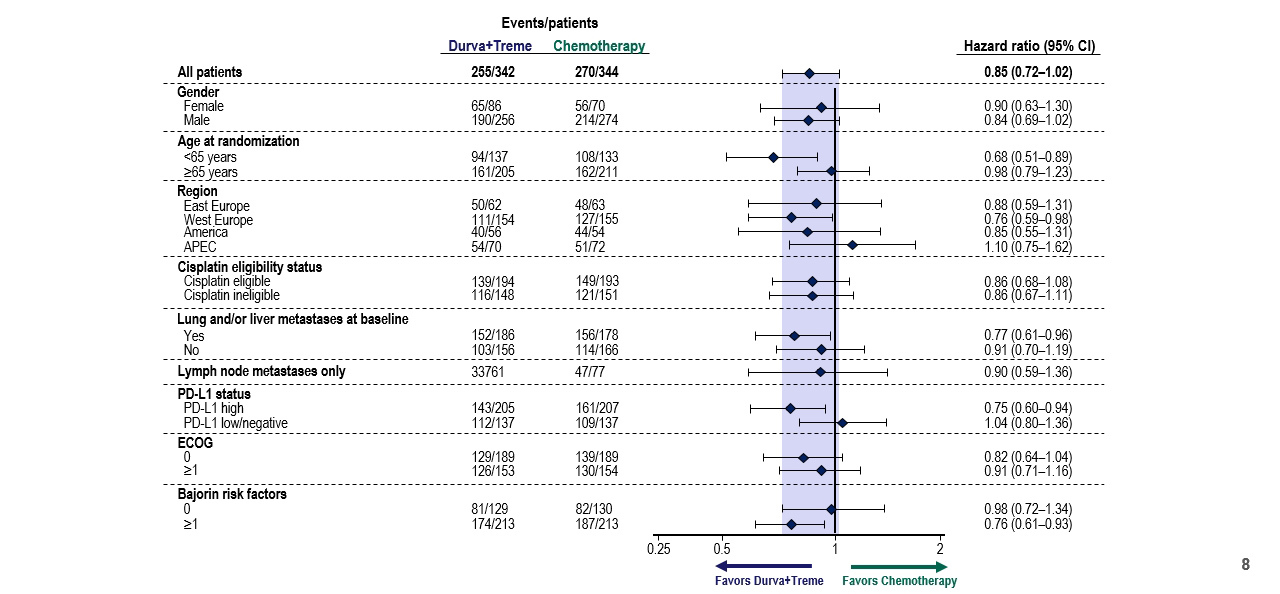
Similar to the other co-primary outcome, subgroup analyses failed to demonstrate meaningful effect modification by patient or disease characteristics. However, there is a suggestion of benefit in younger patients and those with PD-L1 high status.
Exploratory analyses assessing combined immunotherapy versus chemotherapy in PD-L1 positive patients suggested a benefit to the immunotherapy approach with a hazard ratio of 0.4. Thus, while the trial overall demonstrates that chemotherapy should remain the standard of care in first-line therapy, this exploratory analysis suggests that, among a biomarker enriched population, combination immune checkpoint inhibition may be beneficial and may, in the future, supplement chemotherapy in this group.
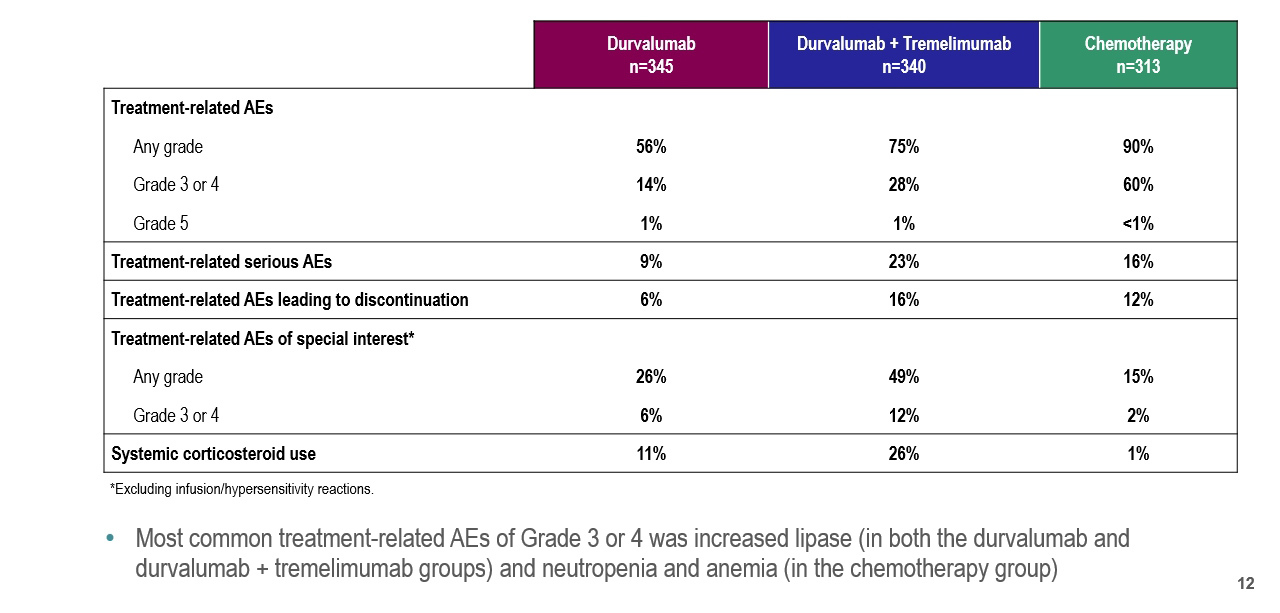
Dr. Powles highlighted that toxicity was more common among patients receiving standard of care chemotherapy than receiving either of the immunotherapy approaches.
In summary, Dr. Powles highlighted that the DANUBE trial failed to meet either of the co-primary endpoints, though the secondary analyses suggested that the combination approach may have benefit in patients with tumors with high PD-L1 expression.
Presented by: Thomas Powles, MBBS, MRCP, MD, Professor of Genitourinary Oncology, Lead for Solid Tumour Research at Barts Cancer Institute, Director of Barts Cancer Institute, London, United Kingdom
Written by: Christopher J.D. Wallis, Urologic Oncology Fellow, Vanderbilt University Medical Center Contact: @WallisCJD on Twitter at the 12th European Multidisciplinary Congress on Urological Cancers (EMUC) (#EMUC20 ), November 13th - 14th, 2020
Related Content:
Durvalumab Plus Tremelimumab for Frontline, Metastatic, Urothelial Cancer, The Phase III DANUBE Trial - Thomas Powles
ESMO Virtual Congress 2020: A Phase 3, Randomized, Open-Label Study of the First-Line Durvalumab with or without Tremelimumab vs. Standard of Care Chemotherapy in Patients with Unresectable, Locally Advanced or Metastatic Urothelial Carcinoma (DANUBE)


