
Dr. Bjartell notes that one of the “game-changer” articles to come out of the EAU Research Foundation was the PRECISION trial, published in The New England Journal of Medicine.1 In PRECISION, PRostate Evaluation for Clinically Important Disease: Sampling Using Image-guidance Or Not? (PRECISION), 500 men with a clinical suspicion of prostate cancer who had not undergone biopsy previously were allocated to undergo MRI, with or without targeted biopsy, or standard transrectal ultrasonography-guided biopsy. Clinically significant cancer was detected in 95 men (38%) in the MRI-targeted biopsy group, as compared with 64 of 248 (26%) in the standard-biopsy group (p=0.005). Fewer men in the MRI-targeted biopsy group than in the standard biopsy group received a diagnosis of clinically insignificant cancer (p<0.001). Dr. Bjartell highlighted that in <2 years since publication, the role of MRI prior to prostate biopsy is already in the guidelines.
A second impactful recently published EAU Research Foundation trial was the NIMBUS trial, Treatment of High-Grade Non-Muscle Invasive Urothelial Carcinoma of the Bladder by Standard Number and Dose of Intravesical BCG Instillations Versus Reduced Number of Intravesical Instillations with Standard Dose of BCG (NIMBUS), for patients receiving Bacillus Calmette-Guerin (BCG) for non-muscle invasive bladder cancer (NMIBC).2 In this trial, 345 patients from 51 sites were randomized between December 2013 and July 2019. The standard BCG schedule was 6 weeks of induction followed by 3 weeks of maintenance at 3, 6, and 12 months (15 instillations). The reduced frequency BCG schedule was an induction at weeks 1, 2, and 6 followed by 2 weeks (weeks 1 and 3) of maintenance at 3, 6, and 12 months (nine instillations). Overall, 170 patients were randomized to reduced frequency and 175 to standard BCG. After a 12 month of median follow-up, the intention-to-treat analysis showed a safety-relevant difference in recurrences between treatment arms: 46/170 (reduced frequency) versus 21/175 patients (standard). Based on these results, the trial concluded that the reduced frequency schedule was inferior to the standard schedule regarding the time to the first recurrence.
A new trial highlighted by Dr. Bjartell is the PEGASUS trial, Safety and Efficacy of Pemigatinib in Patients With High-risk Urothelial Cancer After Radical Surgery (PEGASUS), an open-label, single-arm, phase II study evaluating the safety and efficacy of pemigatinib as adjuvant therapy for molecularly-selected, high-risk patients with urothelial carcinoma who have received radical surgery. Pemigatinib is a selective, potent oral inhibitor of FGFR1, 2, and 3 and has shown to have efficacy in tumors with various FGFR alterations. FGFR genomic alterations are implicated in the pathogenesis of urothelial carcinoma, most commonly FGFR3 mutations (~12%) and translocations (~3-6%). The study design for PEGASUS is as follows:
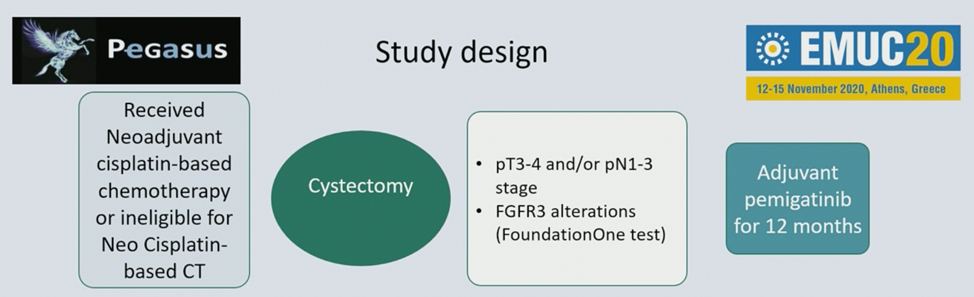
The primary outcome of this trial is relapse-free survival, and secondary outcomes include to evaluate the safety, tolerability, and overall survival. Exploratory outcomes are to evaluate biomarkers of clinical benefit and prognostic biomarkers.
The EAU Research Foundation has also announced a call for prospective multicenter registries, which are as follows:
- eCORE COMET: kidney stones
- Rogue-1: T1 NMIBC
- BRaVeRY: Bladder Variants Registry
- RING: Next-generation imaging
- Frontier: Renal cell carcinoma
As follows are seeding grants that were approved in 2018:
- Penile cancer, PDX models: Maarten Albersen, Leuven
- Prostate cancer, primary tumor removal in oligometastatic disease: Johannes Linxweiler, Hamburg
- Prospective validation of a novel EPS-metabolomic signature in prostate cancer: Nicola Fossati, Milan
- Discovery and functional evaluation of actionable targets in high-risk NMIBC: Tahlita Zuiverloon, Rotterdam
As follows are seeding grants approved in 2019-2020:
- Veeru Kasivisvanathan, London: Prostate imaging using MRI +/- contrast enhancement (PRIME): A study comparing bi-parametric to multi-parametric MRI in the diagnosis of clinically significant prostate cancer
- Melanie Hassler, Vienna: Molecular classification of muscle-invasive bladder cancer patients scheduled for radical cystectomy using a single-sample transcriptomic consensus classifier
- Steven MacLennan, Aberdeen: Imagine – Impact Assessment of Guidelines Implementation and Education: The Next Frontier for Harmonizing Urological Practice across Europe by Improving Adherence to Guidelines
Dr. Bjartell concluded his presentation with several future aims of the EAU Research Foundation:
- Expand the portfolio of investigator-initiated clinical research projects and registries through seeding grants and other set-ups
- Supporting Young Academic Urologists (EAU-YAU)
- Consensus meetings and broad collaborations to fill gaps of knowledge
- Focus on Big Data and real-world data from electronic health records
Presented by: Anders S. Bjartell, MD, Ph.D., FEBU, Skane University Hospital, Malmo, Sweden
Written by: Zachary Klaassen, MD, MSc – Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia Twitter: @zklaassen_md at the 12th European Multidisciplinary Congress on Urological Cancers (EMUC) (#EMUC20 ), November 13th - 14th, 2020
References:
- Kasivisvanathan V, Rannikko AS, Borghi M, et al. MRI-targeted or standard biopsy for prostate cancer diagnosis. N Engl J Med 2018;378(19):1767-1777.
- Grimm MO, van der Hiejden AG, Colombel M, et al. Treatment of High-grade non-muscle-invasive bladder carcinoma by standard number and dose of BCG instillations versus reduced number and standard dose of BCG instillations: Results of the European Association of Urology Research Foundation Randomized Phase III Clinical Trial “NIMBUS”. Eur Urol 2020 Nov;78(5):690-698.


