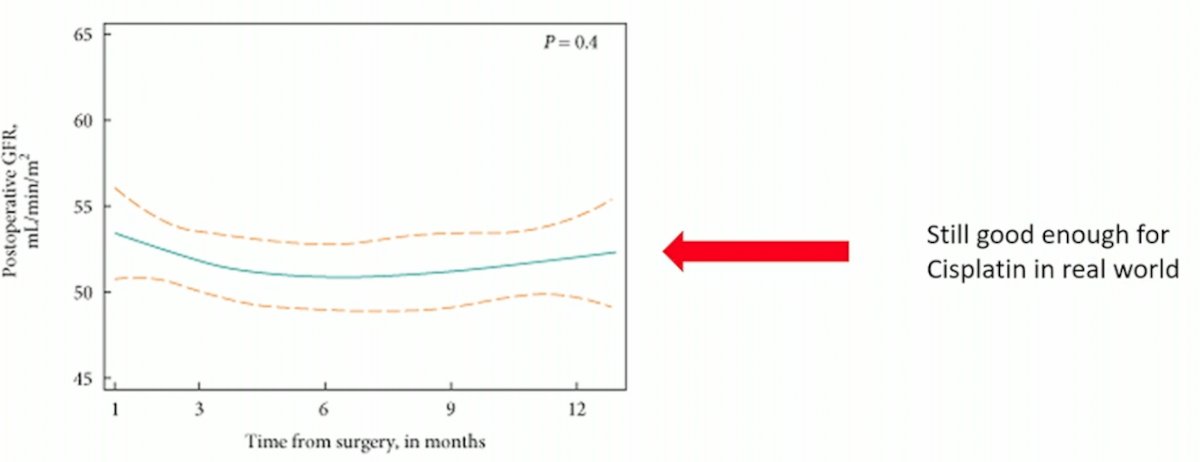
(UroToday.com) The 2023 EAU annual meeting included an EAU guideline session on effective treatment in upper tract urothelial tumors, featuring a state-of-the-art presentation by Dr. Alison Birtle discussing the role of systemic chemotherapy after radical nephroureterectomy. Dr. Birtle started by noting that there are preoperative predictors of renal function decline after radical nephroureterectomy for upper tract urothelial carcinoma, however this is still typically good enough for cisplatin in the real world:
Dr. Birtle’s comments regarding neoadjuvant therapy include: (i) it is difficult to accurately preoperatively diagnosis and stage patients, (ii) there will be over treatment, (iii) there is confusion between neoadjuvant and downstaging chemotherapy, (iv) pathological complete response endpoints are important, and (v) exclusion of carboplatin eligible patients can be an issue. Loew and colleagues [1] previously performed a systematic review and meta-analysis assessing neoadjuvant and adjuvant therapy for upper tract urothelial carcinoma. They found that for neoadjuvant chemotherapy, the pooled pathologic complete response rate (≤ypT0N0M0) was 11% (n = 811) and pathologic partial response rate (≤ypT1N0M0) was 43% (n = 869), both across 14 studies. Across six studies, the pooled HRs were 0.44 (95% CI 0.32-0.59, p < 0.001) for overall survival and 0.38 (95% CI 0.24-0.61, p < 0.001) for cancer-specific survival in favor of neoadjuvant chemotherapy. For adjuvant chemotherapy, there was a benefit in overall survival (pooled HR 0.77; 95% CI: 0.64-0.92, p = 0.004, 7,983 patients), cancer specific survival (pooled HR 0.79; 95% CI: 0.69-0.91, p = 0.001, 5659 patients), and disease-free survival (pooled HR 0.52; 95% CI: 0.38-0.70, 602 patients). As such, Dr. Birtle notes that at best the level of evidence for neoadjuvant chemotherapy is level 2, whereas for adjuvant chemotherapy is level 1 (based on the POUT trial).
Dr. Birtle then discussed the POUT trial, first presented at GU ASCO in 2018 and subsequently published in The Lancet [2] in 2020, followed by an updated analysis presented at GU ASCO 2021. This was a phase III, parallel group, open-label, randomized controlled trial done at 71 NHS hospitals in the UK. Eligible patients had received a radical nephroureterectomy for UTUC, were postoperatively staged with either muscle-invasive (pT2–pT4, pNany) or lymph node-positive (pTany, pN1–3) M0 disease with predominantly transitional cell carcinoma histology, and were fit to receive adjuvant chemotherapy within 90 days of surgery. Patients also had to have a glomerular filtration rate (GFR) of ≥30 mL/min. Patients were randomized 1:1 to receive either surveillance or adjuvant chemotherapy: four 21-day cycles of platinum-based chemotherapy (cisplatin 70 mg/m2) within 14 days of randomization; gemcitabine (1000 mg/m2) given on days 1 and 8 of each cycle. Patients with impaired renal function (GFR ≥30 mL/min and <50 mL/min) received carboplatin rather than cisplatin. The primary endpoint of this trial was DFS, and secondary endpoints included metastasis-free survival (MFS), OS, treatment compliance, acute toxicity, late toxicity, and patient-reported quality of life.
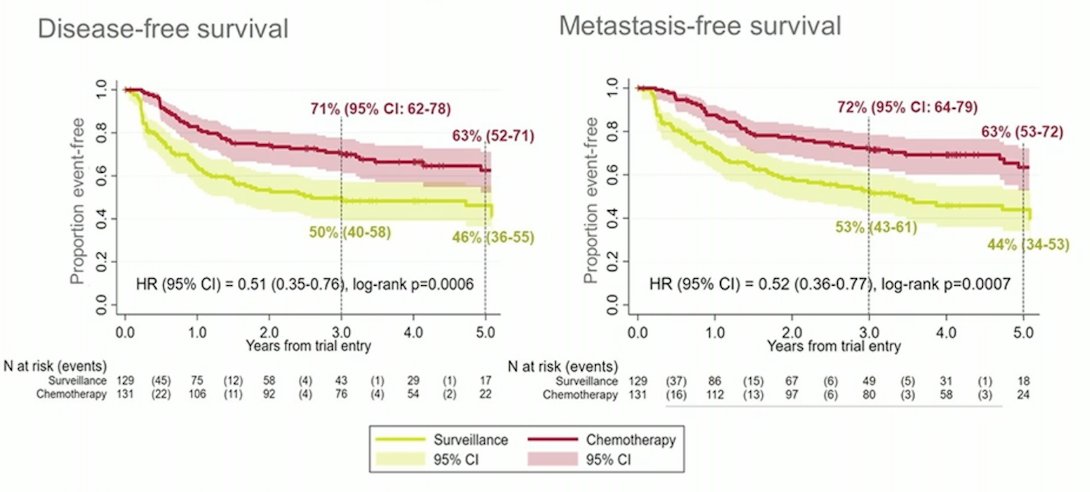
There were 261 patients included in the trial between June 19, 2012 and November 8, 2017, including 129 patients randomized to surveillance and 132 to chemotherapy; 260 patients were included in the intention to treat analysis. There were 60 (47%) DFS events in the surveillance cohort and 35 (27%) in the chemotherapy cohort; as such, the unadjusted HR was 0.45 (95%CI 0.30-0.68) in favor of chemotherapy (log-rank p = 0.0001). The three year DFS rate was 46% for surveillance (95%CI 36-56) and 71% for chemotherapy (95%CI 61-78). MFS also favored chemotherapy, with a HR of 0.48 (95%CI 0.31-0.74, log-rank p = 0.0007), and the three-year event-free rates were 53% (95% CI 42-63) for those on surveillance and 71% (95% CI 60-79) for those receiving chemotherapy. At the time of the 2021 GU ASCO 2021 update, there were 64/129 DFS events in the surveillance arm compared to 45/131 in the chemotherapy arm (HR 0.54, 95% CI 0.36-0.79). For MFS, there were 66/129 events in the surveillance arm and 45/131 in the chemotherapy arm (HR 0.55, 95% CI 0.37-0.82), thus both endpoints showing continued benefit for chemotherapy:
With regards to overall survival, the trial was not powered for this endpoint and since POUT met its primary endpoint of DFS, the trial was closed early. However, GU ASCO 2021 was the first presentation of mature OS data, with 52/129 events in the surveillance arm compared to 41/131 in the chemotherapy arm (HR 0.77, 0.50-1.17). Post-recurrence (local or metastatic) systemic therapy was received in 45 patients in the surveillance arm and 18 in the chemotherapy arm.
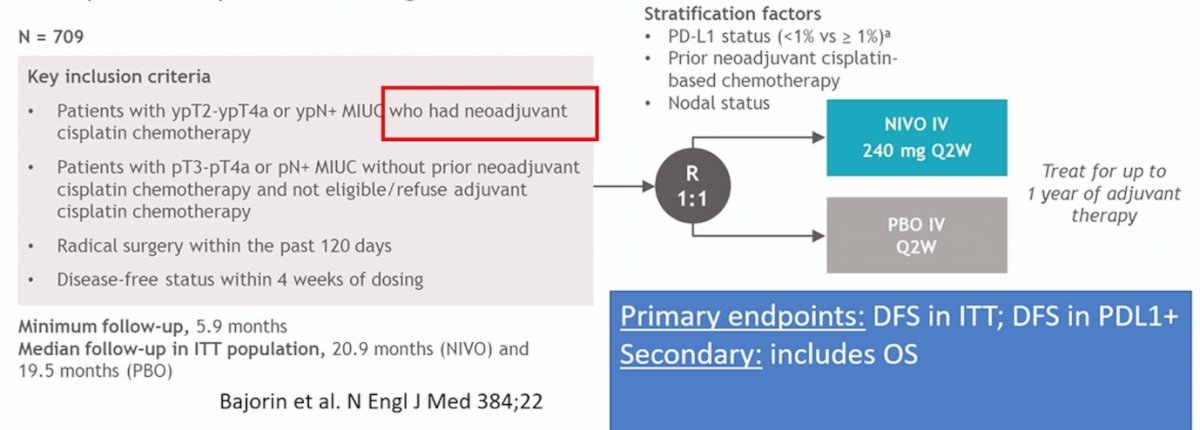
Dr. Birtle then highlighted that there are additional trials to consider, including CheckMate 274 (adjuvant nivolumab vs placebo) [3], Ambassador (adjuvant pembrolizumab vs observation), and Uranus (radical nephroureterectomy if cisplatin unfit vs neoadjuvant [both groups platinum unfit]). The CheckMate 274 trial is a phase 3, randomized, double-blind, multicenter study of adjuvant nivolumab versus placebo in patients with high-risk muscle invasive urothelial carcinoma. The trial design for CheckMate 274 is as follows:
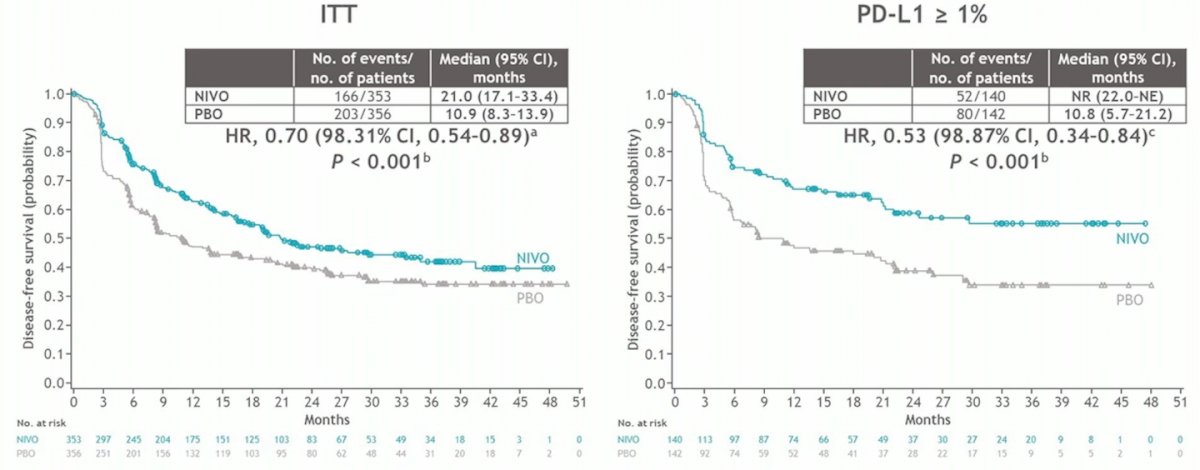
This was a positive trial, with a disease free survival benefit in both the intention to treat analysis and the PD-L1 > 1% analysis:
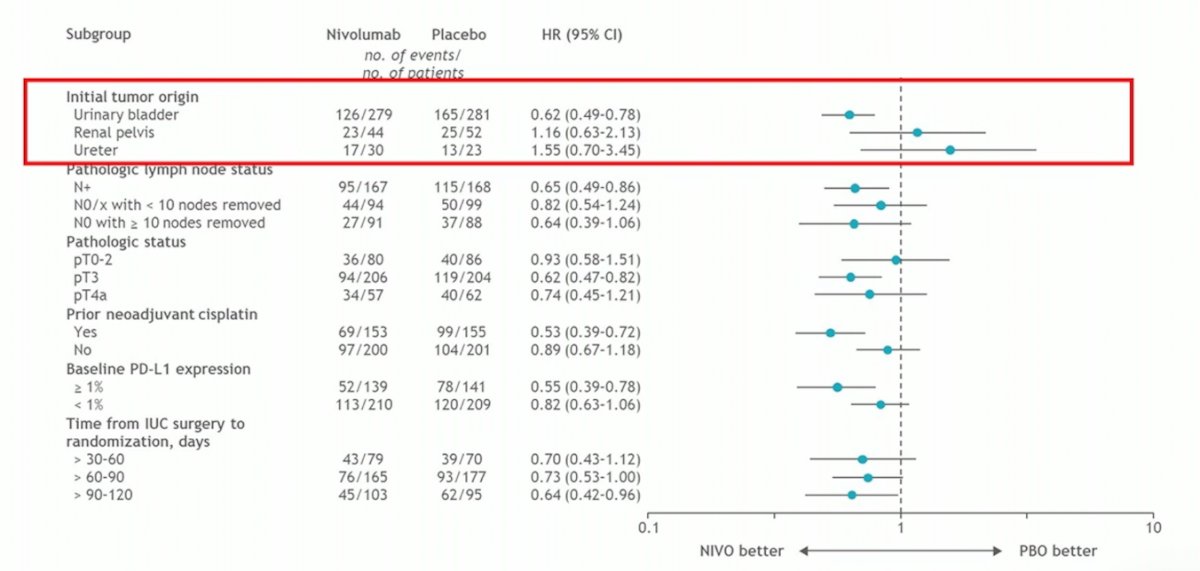
Of note, overall survival currently has insufficient events and thus no data for survival has been presented to date. CheckMate 274 also included patients with ureteral and renal pelvis tumors, however by subgroup analysis, there was no benefit to nivolumab:
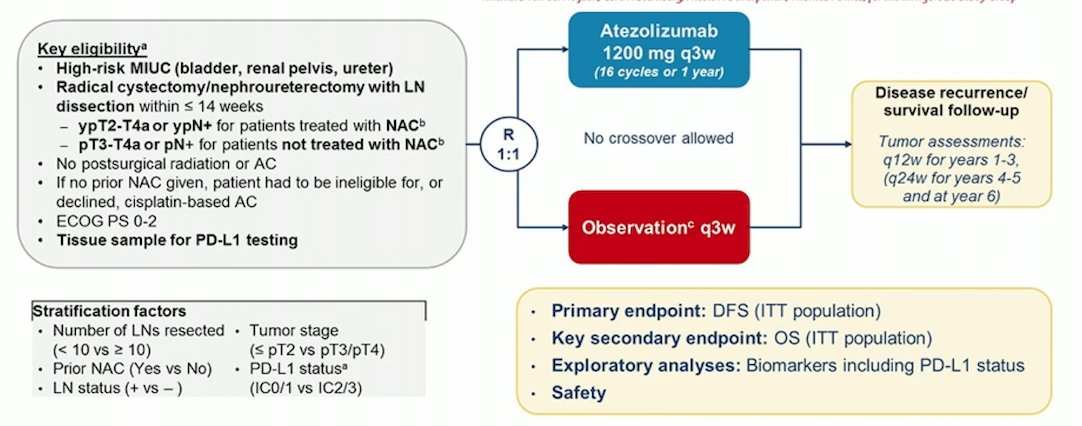
The IMvigor010 trial [4] randomized patients with muscle invasive urothelial carcinoma to adjuvant atezolizumab versus observation, with the following trial design:
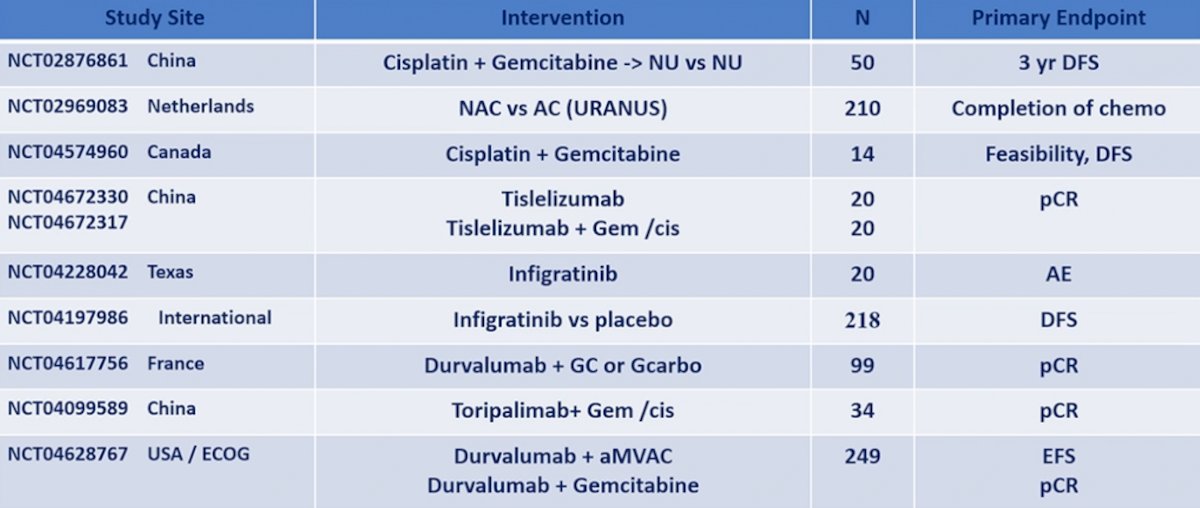
Unfortunately, this was a negative trial, with no DFS benefit in the intention to treat population (HR 0.89, 95% CI 0.74-1.08). Finally, the PROOF 302 trial was set to assess adjuvant infigratinib versus placebo for invasive urothelial carcinoma with susceptible FGFR3 alterations, but this trial has subsequently been withdrawn. As follows is a list of ongoing and future trials in pre-operative therapy for upper tract urothelial carcinoma:
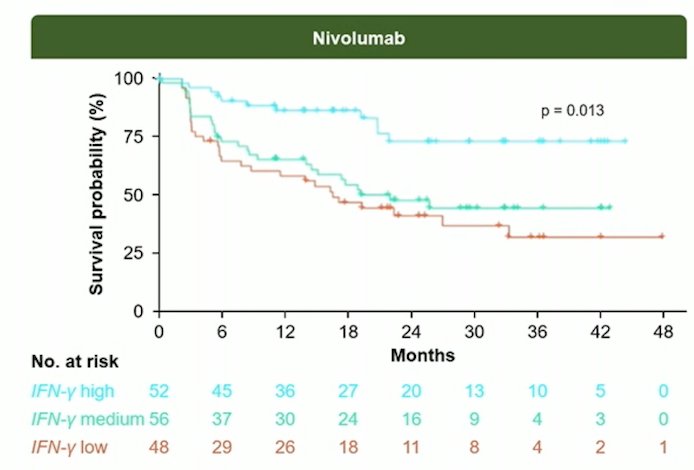
Dr. Birtle emphasized that from here we are likely heading towards biomarkers, using samples and date collected in CheckMate 274 and POUT. For example, in CheckMate 274, the IFN-gamma gene signature was associated with higher DFS in nivolumab treated patients (p = 0.013):
Dr. Birtle concluded her presentation discussing the role of systemic chemotherapy after radical nephroureterectomy with the following take-home messages:
- Upper tract urothelial carcinoma is a low incidence tumor, but does have a DFS benefit for adjuvant chemotherapy for high-risk disease
- Biomarkers are not yet ready for everyday use
- For adjuvant immunotherapy, why is one different to the other for DFS?
- The POUT trial offers phase III, level 1 evidence for adjuvant chemotherapy
Presented by: Alison J. Birtle, MD, Rosemere Cancer Centre, Lancashire Teaching Hospitals, Preston, United Kingdom
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2023 European Association of Urology (EAU) Annual Meeting, Milan, IT, Fri, Mar 10 – Mon, Mar 13, 2023.
References:
- Leow JJ, Chong YL, Chang SL, et al. Neoadjuvant and Adjuvant Chemotherapy for Upper Tract Urothelial Carcinoma: A 2020 Systematic Review and Meta-Analysis, an Future Perspectives on Systemic Therapy. Eur Urol. 2021 May;79(5):635-654.
- Birtle A, Johnson M, Chester J, et al. Adjuvant chemotherapy in upper tract urothelial carcinoma (the POUT trial): A phase 3, open-label, randomized controlled trial. Lancet 2020 Apr 18;395(10232):1268-1277.
- Bajorin DF, Witjes JA, Gschwend JE, et al. Adjuvant nivolumab versus placebo in muscle-invasive urothelial carcinoma. N Engl J Med. 2021 Jun 3;384(22):2102-2114.
- Bellmunt J, Hussain M, Gschwend JE, et al. Adjuvant atezolizumab versus observation in muscle-invasive urothelial carcinoma (IMvigor010): A multicentre, open-label, randomized, phase 3 trial. Lancet Oncol. 2021 Apr;22(4):525-537.