(UroToday.com) The 2022 EAU annual meeting featured a session on improvements in metastatic prostate cancer focusing on imaging and treatment, including a presentation by Dr. Bertrand Tombal discussing results from the phase 3 HERO study, specifically sustained castration to < 20 ng/dl for relugolix versus leuprolide in men with advanced prostate cancer. Achieving testosterone level of <20 ng/dL (≤0.7 nmol/L) for castration is associated with improved overall and cancer specific survival outcomes.1 In the phase 3 HERO study, the oral GnRH receptor antagonist, relugolix, demonstrated improved suppression of testosterone to profound castrate levels on Day 15 versus leuprolide (78.4% vs. 1.0%) in men with advanced prostate cancer.2 At the 2022 EAU meeting, Dr. Tombal provided an in-depth analysis of the assessment of profound castrate levels (< 20 ng/dL [≤ 0.7 nmol/L]) from the HERO study.
Overall, there were 934 men that were randomized 2:1 to receive relugolix 120 mg orally once daily after a single oral loading dose of relugolix 360 mg on Day 1 or leuprolide injections every 12 weeks for 48 weeks. Profound castration rate, defined as the cumulative probability of testosterone suppression to <20 ng/dL prior to dosing at Day 15, was summarized by treatment group using the Kaplan-Meier method:

Evaluation of the time course and magnitude of sustained profound castration throughout the study were also assessed. Time to initial profound testosterone castration was defined as time from the date of first dose to the date of initial testosterone suppression to <20 ng/dL. All analyses were conducted in a modified intent to treat population.
A total of 622 men received relugolix and 308 received leuprolide. The median time to profound castration was 15 days (95% CI NE, NE) in the relugolix group and 29 days (95% CI NE, NE) in the leuprolide group:
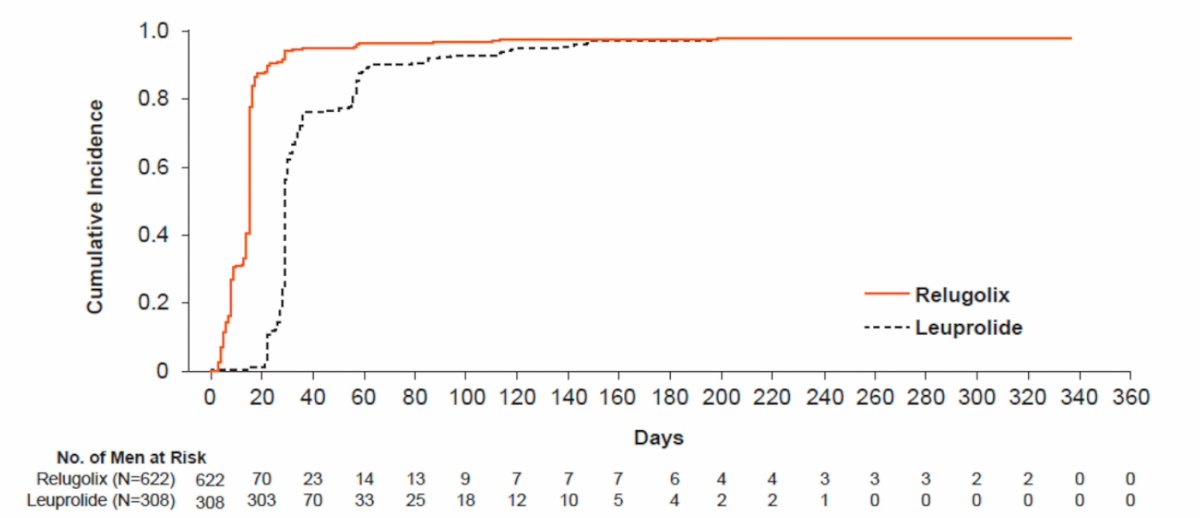
At day 15, the profound castration rate was 78.4% (95% CI 75.1%, 81.5%) in the relugolix group and 1.0% (95% CI 0.3%, 3.0%) in the leuprolide group. Sustained profound castration was higher for the relugolix group compared with the leuprolide group at all measured time points, with Day 337 (48 weeks) profound castration rates of 81.6% (95% CI: 78.1%, 84.5%) and 68.6% (95% CI: 63.0%, 73.5%) in the relugolix and leuprolide groups, respectively:

Dr. Tombal concluded his presentation by discussing the results of sustained castration to < 20 ng/dl for relugolix versus leuprolide in men with advanced prostate cancer emphasizing that relugolix demonstrated more rapid and sustained profound testosterone suppression compared with leuprolide in the phase 3 HERO study at all measured time points.
Presented by: Bertrand Tombal, MD, PhD, Université Catholique de Louvain, Institut de Recherche Clinique, Brussels, Belgium
Co-Authors: Shore N.D.2, George D.J.3, Cookson M.S.4, Saltzstein D.R.5, Mehlhaff B.6, Tutrone R.7, Bailen J.L.8, Brown B.9, Lu S.10, Schulmann T.11, Hanson S.12, Saad F.13
Affiliations: 2Carolina Urologic Research Center, Dept. of Urology, Myrtle Beach, United States of America, 3Duke University, Duke Cancer Institute Center for Prostate and Urologic Cancers, Durham, United States of America, 4University of Oklahoma, The University of Oklahoma Health Sciences Center, Oklahoma City, United States of America, 5Urology San Ant, Dept. of Urology, San Antonio, United States of America, 6Oregon Urology Institute, Dept. of Urology, Springfield, United States of America, 7Chesapeake Urology, Dept. of Urology, Towson, United States of America, 8First Urology, Dept. of Urology, Jeffersonville, United States of America, 9Myovant Sciences, Inc., Brisbane, United States of America, 10Myovant Science, Inc., Brisbane, United States of America, 11Myovant Sciences, GmbH, Basel, Switzerland, 12Pfizer, Inc., New York, United States of America, 13University of Montreal, University of Montreal Hospital Centre, Montreal, Canada
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2022 European Association of Urology (EAU) Annual Hybrid Meeting, Amsterdam, NL, Fri, July 1 – Mon, July 4, 2022.
References:
- Klotz L, Breau RH, Collins LL, Gleave ME, et al. Maximal testosterone suppression in the management of recurrent and metastatic prostate cancer. Can Urol Associ J. Jan-Feb 2017;11(1-2):16-23.
- Shore ND, Saad F, Cookson MS, et al. Oral Relugolix for Androgen-Deprivation Therapy in Advanced Prostate Cancer. N Engl J Med. 2020 Jun 4;382(23):2187-2196.


