- Hormone/Castration sensitive vs. Hormone/Castration naïve: some men have prior adjuvant hormonal therapy (not all are naïve), and the patient community does not like the word “castration”. Dr. Sweeney’s preferred term is metastatic hormone-sensitive prostate cancer (mHSPC)
- Androgen deprivation therapy (ADT) or hormone therapy: There are many versions of testosterone suppression, including testosterone alone, with a weak or potent androgen receptor inhibitor (an ‘amide), or with extragonadal androgen synthesis inhibition (abiraterone)
- There are different versions of monotherapy and combined androgen blockade
Definitions matter when used to guide the counselling and treatment of patients, whether it is prognostic and/or predictive. “Oligometastatic” has generated much interest and investigation: at the APCCC19 meeting, 79% of the expert consensus voted to be able to deliver ablative therapy with curative intent/hope.1 “Poly-metastatic” disease is high volume (>3 bone mets and/or liver mets) vs low volume (extensive nodes not amenable to surgery or radiotherapy). Indeed, there are many versions of mHSPC, including de novo presentation (high vs low volume) or metachronous presentation (high vs low volume).
Ultimately, patients with mHSPC have variable outcomes with testosterone suppression. Combining data from CHAARTED and GETU15 (high volume disease being defined as visceral metastases and/or 4 or more bone metastases with at least one beyond the vertebra and pelvis), the median OS outcomes are as follows:
- Prior local therapy and low volume disease: ~8 years
- Prior local therapy and high volume disease: 4.5 years
- De novo and low volume disease: 4.5 years
- De novo and high volume disease: 3 years
The spectrum of patients with mHSPC varies – one extreme is the 55-year-old man with no comorbidities and high-volume de novo metastatic disease, and the other extreme is the 82-year-old man with CHF and CAD with two bone metastases 10 years after a radical prostatectomy. Indeed, these two examples call for different treatment paradigms. Dr. Sweeney’s guidance for polymetastatic disease in 2020 is as follows:
- Docetaxel versus an ‘amide versus abiraterone depends on fitness for chemotherapy, fitness for apalutamide/enzalutamide (no seizure, no frailty), and fitness for abiraterone (blood sugar, hypertension, liver function)
- Evidence of consistent overall survival benefit for a given setting with use of a given agent with no decrement in quality of life on therapy
- If chemofit and high volume (and not in the COVID pandemic): docetaxel should be considered at the time of testosterone suppression or at CRPC after abiraterone or an ‘amide, and we should consider giving docetaxel first when the patient is most fit
- If chemofit and low volume disease not amenable to surgery or radiotherapy: there is consistent evidence for an ‘amide/abiraterone, with less consistency for docetaxel (but don’t forget about docetaxel for mCRPC)
As follows is a high-level summary of all data for testosterone suppression +/- docetaxel for mHSPC: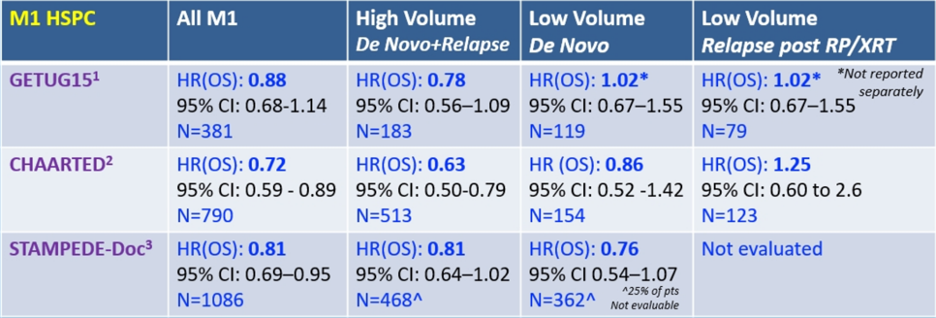
Dr. Sweeney encourages a closer look at the fine print behind the “mixed” results:
- GETUG15 – was conducted in an era with minimal access to agents other than docetaxel for CRPC. Furthermore, this data shows that the Kaplan Meier curves for de novo low volume disease are overlapping and is based on retrospective scan data on all patients. Importantly, the outcomes of low and high volume subgroups are homogeneous with CHAARTED
- CHAARTED – is the only study with prospective stratification by volume of disease
- STAMPEDE – is missing scan data on 24% of the patients in retrospective volume analysis and does not include any patients with metachronous low volume disease
The bottom line according to Dr. Sweeney is that probably some patients with low volume de novo metastatic disease do have a benefit from chemotherapy but not large enough to see a clear treatment effect across all trials. Additionally, even patients with high volume disease do not necessarily derive a benefit from chemotherapy, thus we need biomarkers to personalize treatment. Two additional points are that (i) it is unknown if there is any treatment effect with docetaxel (or the ‘amides/abiraterone) in low volume disease after radiation to the primary if the patient is de novo metastatic, and (ii) there is no data to suggest any benefit with docetaxel in metachronous low volume disease.
Looking at abiraterone by volume of disease, the LATITUDE study (with the CHAARTED definition for volume) showed a benefit in high volume disease for abiraterone (HR 0.62, 95% CI 0.52-0.74), but no benefit for low volume patients (HR 0.72, 95% CI 0.47-1.10). Looking at the STAMPEDE abiraterone arm, patients receiving abiraterone had significantly improved OS (low-risk hazard HR 0.66, 95% CI 0.44-0.98) and failure-free survival (low-risk HR 0.24, 95% CI 0.17-0.33) compared with ADT alone.2 Additionally, heterogeneity of effect was not seen between low- and high-risk groups for overall survival or failure-free survival. Enzalutamide in the ENZAMET study3 showed an OS benefit for enzalutamide (HR 0.67, 95% CI 0.52-0.85), but is a bit of a mixed bag with regards to de novo vs metachronous, the fact that many patients received concurrent docetaxel and other permutations. At the interim analysis, there was a clear OS benefit in low volume patients and patients who did not get docetaxel; 71% of patients with high volume disease got docetaxel, thus less of an enzalutamide benefit with short-term follow-up (HR 0.80, 95% CI 0.59-1.07). Among the patients with low volume disease, 37% received docetaxel, and there was a clear benefit to enzalutamide (HR 0.43, 95% CI 0.26-0.72)
As follows is an excellent summary of treatment benefit for overall survival in mHSPC: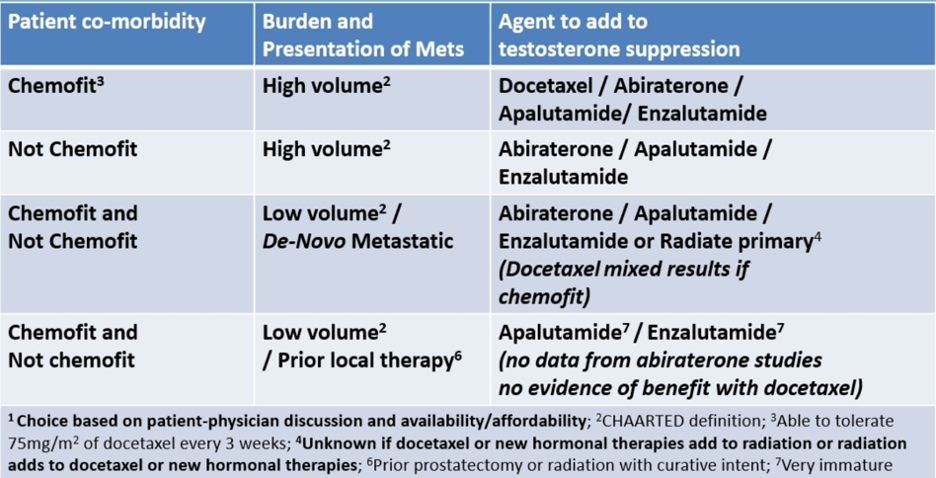
Dr. Sweeney concluded that when deciding on which systemic therapy for which patient with mHSPC, it is important to:
- Decide if they are chemo-fit or not chemo-fit
- Delineate whether they have a low or high burden of mHSPC
- Look at all of the data to define treatment burden versus treatment benefit for a given subgroup
- Engage the patient in treatment choice: some chemo-fit patients with high volume disease may want to get chemotherapy out of the way; some patients may want to avoid chemotherapy in the COVID pandemic; if important to avoid (eg. chemophobia or hormonophilia)
Presented by: Christopher Sweeney, MBBS, Dana Farber Cancer Center, Boston, MA
References:
- Gillessen S, Attard G, Beer TM, et al. Management of Patients with Advanced Prostate Cancer: Report of the Advanced Prostate Cancer Consensus Conference 2019. Eur Urol 2020 Apr;77(4):508-547.
- Hoyle AP, Ali A, James ND, et al. Abiraterone in “high-“ and “low-risk” metastatic hormone-sensitive prostate cancer. Eur Urol 2019 Dec;76(6):719-728.
- Davis ID, Martin AJ, Stockler MR, et al. Enzalutamide with Standard First-Line Therapy in Metastatic Prostate Cancer. N Engl J Med 2019 Jul 11;381(2):121-131.


