Following introductory remarks and talks from Dr. Cooperberg on Pre-diagnostic testing and germline testing and from Dr. Wagner on how to incorporate testing with Decipher and ProMark at the time of diagnosis, Dr. Lin discussed how to incorporate Prolaris and Oncotype at the time of diagnosis.
He began by highlighting the challenge we have in prostate cancer: NCCN breast cancer guidelines demonstrate highly stratified and personalized treatment recommendations on the basis of hormone receptor status, HER2 status, and 21-gene RT-PCR findings. This progress in other tumor types highlights that molecularly targeted therapy is an achievable reality.
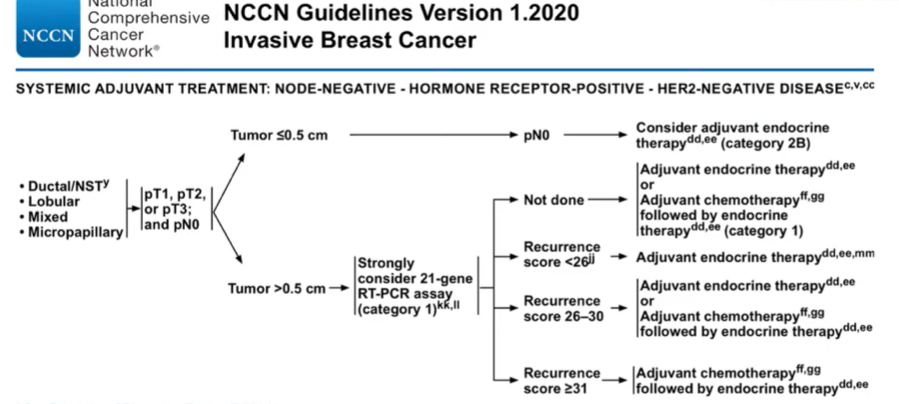
In contrast, in prostate cancer, biomarkers do not form the basis of any guideline-driven treatment recommendations.
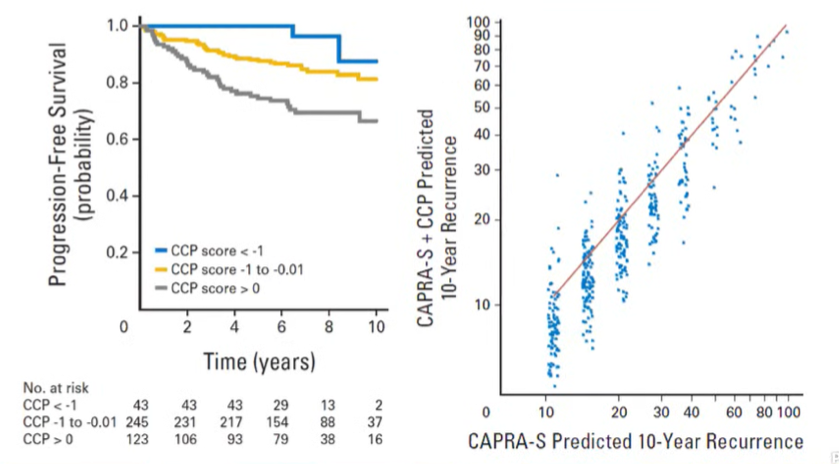
In this context, he began to discuss the potential role of Prolaris. This is a 31-gene cell cycle progression signature based on formalin-fixed biopsy tissue. Notably, Prolaris has published evidence supporting its role in prognosticating prostate cancer-specific mortality in patients on watchful waiting, biochemical recurrence following radical prostatectomy, and for initial treatment decision making. In one of the seminal articles supporting Prolaris, the results of the Prolaris test are able to provide more granular risk prediction within the highly variable rates of recurrence based on clinicopathologic characteristics (as highlighted in the figure to the right below).
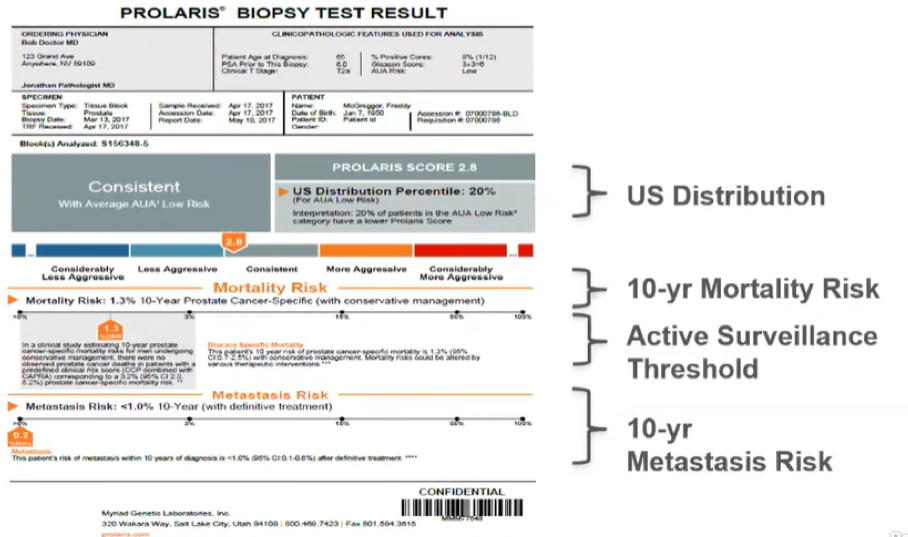
As Dr. Wagner had done, Dr. Lin provided an example of the Prolaris test results, emphasizing results including the US distribution of Prolaris scores for patients with the same AUA risk group, the active surveillance threshold, and the 10-year metastasis risk.
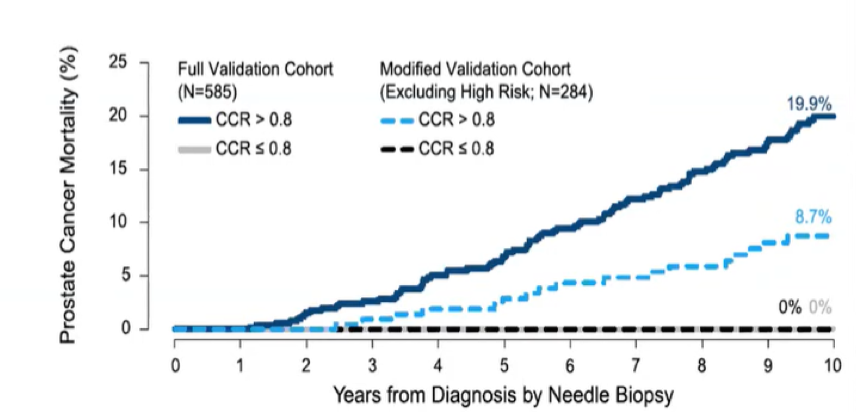
Among men who were candidates for active surveillance, no patients with CCR scores less than 0.8 died of prostate cancer. This was re-capitulated even when those with high-risk characteristics were excluded. Thus, when operationalized in a binary fashion, Prolaris allows for the identification of patients with indolent disease for whom active surveillance is safe.
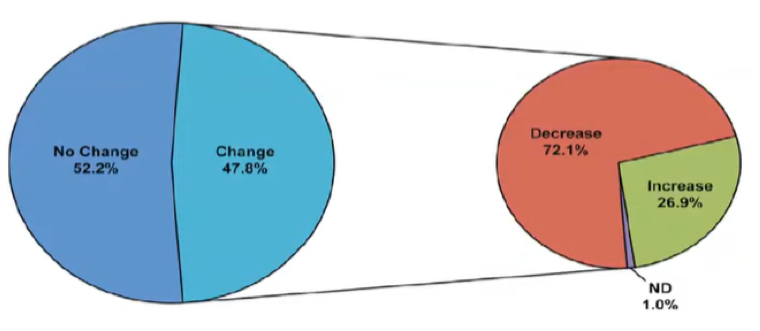
Further data have shown that the use of Prolaris may meaningfully affect treatment decisions in 65% of patients, with the majority of those de-escalating care (40%). Across all AUA risk categories, the use of the Prolaris assay affected decision making. In a second analysis assessing the same question, the use of the Prolaris assay resulted in a change in management in 48% of patients with 72% of those changing management having a decreased intensity of therapy.
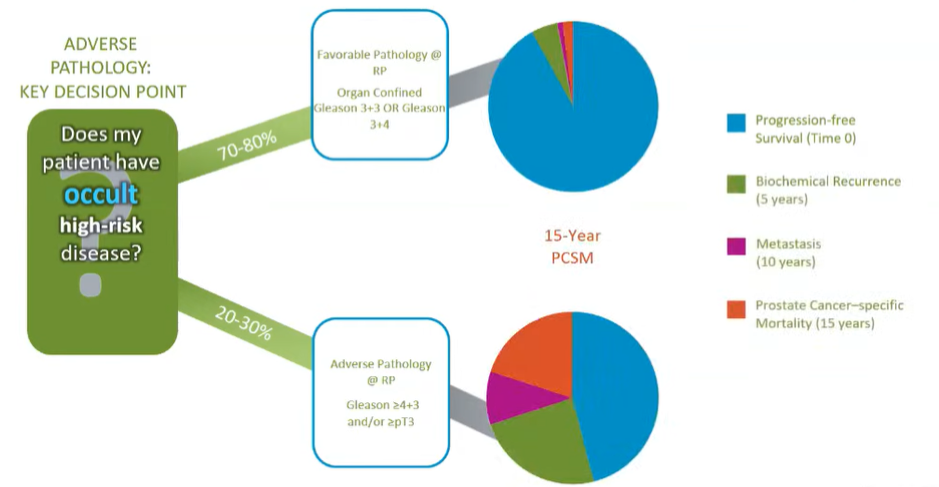
Dr. Lin then reflected on the context of genomic test development and utilization, questioning whether additional tests are needed in patients with low-volume Gleason 3+3 disease for whom active surveillance would be the default treatment recommendation. Further, he wondered whether prostate cancer-specific mortality and metastasis are appropriate and relevant outcomes in these patients given their infrequency. As a result of considerations such as these, he highlighted the ASCO guideline on Molecular Biomarkers in Localized Prostate Cancer which concluded that the use of tissue-based molecular biomarkers should be used “only in situations in which the assay results, when considered as a whole with routine clinical factors, are likely to affect a clinical decision” but not in routine use. Highlighted examples of where molecular assays may be useful include patients with high-volume GG1 disease, with GG1 disease with an abnormal DRE or high PSA density, with low-volume GG2 disease, with adverse pathology at the time of radical prostatectomy, and undetectable PSA. Further, the guideline panel stated that routine use of these assays was limited but the lack of prospective testing and lack of demonstrated improvement in long-term outcomes.
However, molecular assays, as Dr. Lin pointed out, may allow for assessment of the “true nature” of a tumor which is not apparent on the basis of clinical features. Adverse pathology was selected as the outcome of interest on the basis that this was an immediate and actionable endpoint, associated with important long-term outcomes.
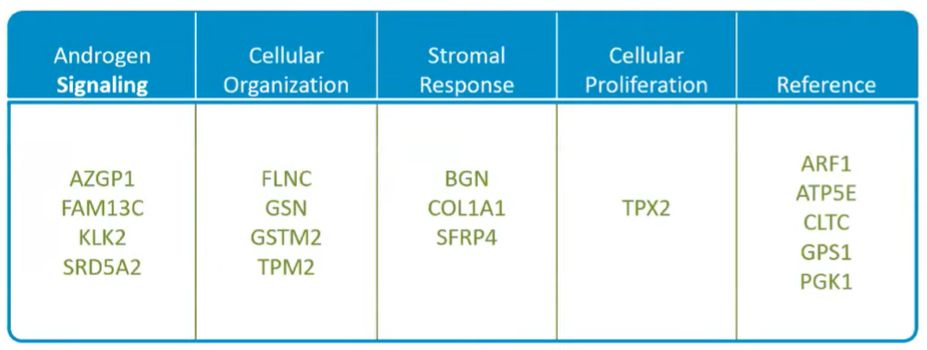
Dr. Lin then highlighted that the Oncotype DX GPS was, in development, based on 727 cancer-specific genes, quantified based on the primary and highest Gleason patterns on radical prostatectomy specimens. The assay was then optimized for biopsy tissue and the list of included genes was narrowed down to those that remained predictive regardless of sampled Gleason pattern. The final GPS comprised a number of genes across multiple pathways, incorporating reference housekeeping genes.
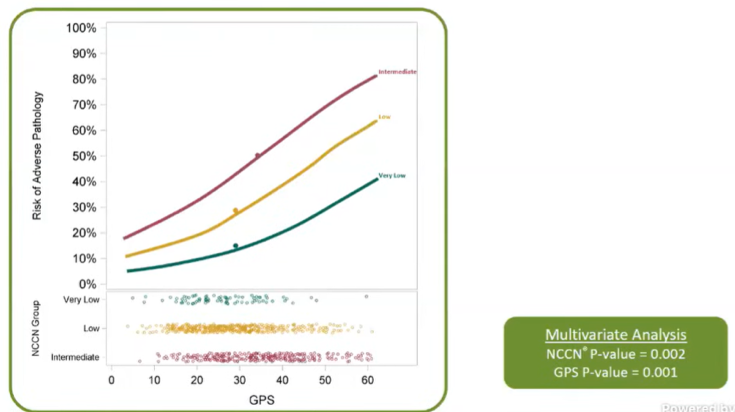
This assay was developed and validated across a number of studies, each comprising hundreds of patients. Meta-analysis of the validation studies demonstrated that GPS is an independent predictor of adverse pathology.
While these data can allow for the identification of those for whom it is safe to defer treatment by taking an active surveillance approach, it is important to understand that this is theoretical because the test was derived and validated on men who have all undergone radical prostatectomy. In addition to the primary endpoint of adverse pathology, subsequent validation studies have shown that GPS is a validated predictor of metastasis and prostate cancer-related mortality.
Clinical utility studies have shown that the use of GPS increases the utilization of active surveillance, particularly in men with NCCN low-risk disease.
Summarizing the data for Oncotype DX GPS, Dr. Lin concluded that GPS adds prognostic information beyond clinicopathologic data and is validated as a predictor of adverse pathology (which is most actionable for patients with low-risk disease) as well as of metastasis and prostate cancer-related mortality.
As with the other assays, Dr. Lin then provided examples of the results of Oncotype DX GPS testing. These results provide similar output to the reports of other assays. Notably, the top-line results integrate GPS results and clinical characteristics to provide a single composite risk estimate
Integrating the entirety of his presentation, Dr. Lin concluded that both Prolaris and Oncotype are tissue-based molecular assays that are independently associated with disease biology and risk stratify patients beyond what is possible using clinicopathologic variables. The use of these tests may increase the utilization of active surveillance, a desirable outcome, supported by clinical utility studies. However, there is a lack of prospective testing and the endpoints employed are somewhat questionable for low-risk patients.
Presented by: Daniel Lin, MD, Associate Professor, Public Health Sciences Division, Fred Hutch, Professor of Urology, Pritt Family Endowed Chair in Prostate Cancer Research, Vice-Chair of Research, Department of Urology, University of Washington, Seattle, Washington.
Written by: Christopher J.D. Wallis, Urologic Oncology Fellow, Vanderbilt University Medical Center, @WallisCJD on Twitter during the AUA2021 May Kick-off Weekend May 21-23


