Two clinical trials were conducted to assess potential pharmacological interaction between vibegron, tolterodine and metoprolol. Vibegron is a novel beta-3 agonist developed for the treatment of overactive bladder (OAB). In vitro data confirm that vibegron does not inhibiy CYP enzymes, thus reducing risk of CYP based drug interactions.
The aim of both trials was to evaluate safety, tolerability, and pharmacokinetics of vibegron in relation to the CYP2D6 substrates due to previous research showing that 43% of patients who take OAB medications are on the medications metabolized through the CYP2D6 pathway.
Healthy volunteers were recruited to participate in two single center open-label clinical trials. Twelve participants were enrolled into double blind, double dummy, randomized and placebo controlled received 4 mg of extended release tolterodine with or without 100 mg of vibegron (Figure 1).
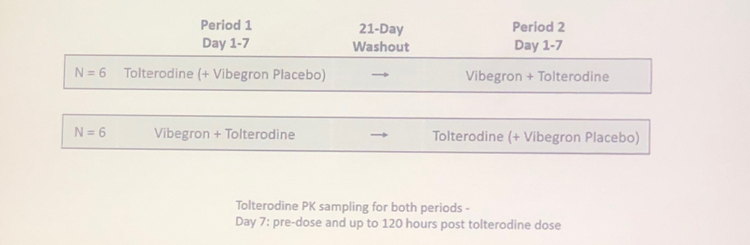
Figure 1
Data analysis demonstrated that vibegron doesn’t affect the plasma concentration of tolterodine, sensitive CYD2P6 substrate (Figure 2).
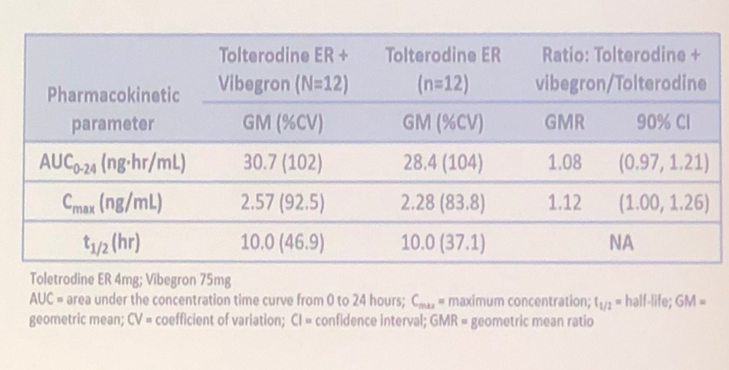
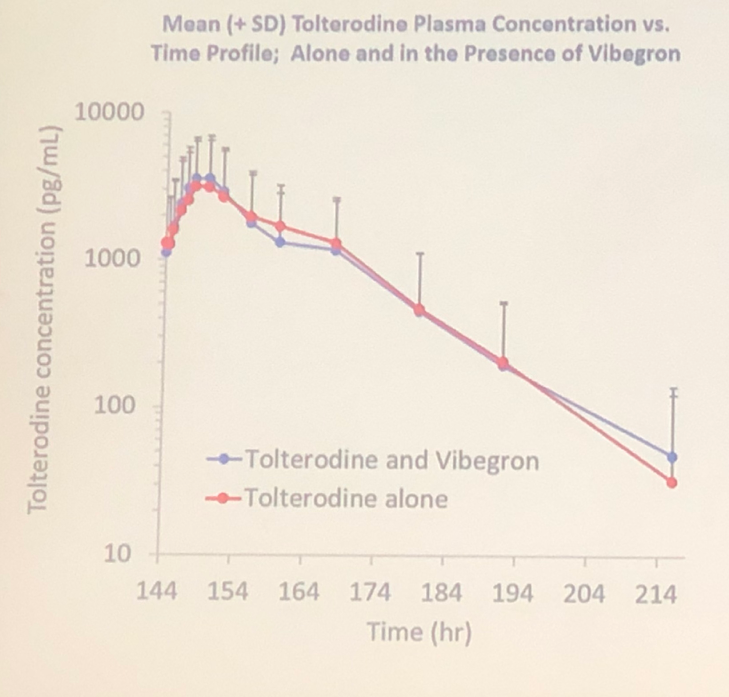
Figure 2
Twenty-four subjects were recruited into the open label, single sequence study of 100 mg of metoprolol succinate with or without 75 mg of vibegron (Figure 3). Drug interactions and adverse events were documented for the duration of the trials.

Figure 3
Research demonstrated slight increase in metoprolol PK levels (Figure 4). Although metoprolol maximum plasma concentration levels increased in subjects exposed to the vibegron, the elimination half-life of metoprolol was comparable to the group who took metoprolol without vibegron. No significant adverse events were noted throughout the trials.
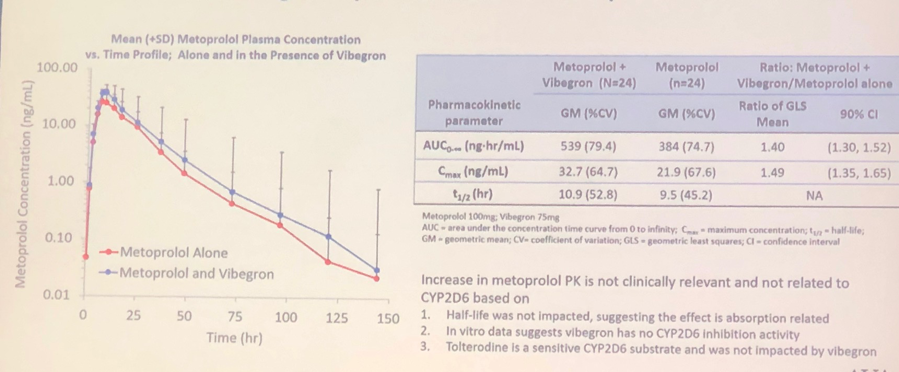
Figure 4
Study demonstrated vibegron doesn’t create a significant drug interaction when it’s taken with tolterodine and metoprolol succinate. It was deemed afe and well-tolerated in healthy volunteers. Vibegron doesn’t affect CYP2D6 pathway and can be utilized by the patients with OAB without contributing to the harmful drug-drug interactions.
Presented by: Matthew P. Rutman, MD, Columbia University College of Physicians and Surgeons
Co-authors: Jennifer R King, Nate Bennett, Durham, NC, Wendy Ankrom, Kenilworth, NJ, Paul N Mudd, Durham, NC
Written by: Hanna Stambakio, BS, Clinical Research Coordinator, Division of Urology, University of Pennsylvania, Twitter: @AStambakio at the American Urological Association's 2019 Annual Meeting (AUA 2019), May 3 – 6, 2019 in Chicago, Illinois


