(UroToday.com) The 2022 ASTRO annual meeting featured a prostate cancer session, including a presentation by Dr. Nina Pujol discussing the Single Fraction Early Prostate Irradiation (SiFEPI) phase II trial assessing single fraction high-dose rate (HDR) brachytherapy for prostate cancer. HDR brachytherapy could allow better tumor control by offering higher doses per fraction and greater optimization of dose distribution. Based on consistent rationale and promising early results reporting oncologic outcomes of a single dose of 19 Gy HDR brachytherapy,1 Dr. Pujol and colleagues conducted the SiFEPI phase 2 prospective trial.
The SiFEPI trial (NCT02104362) evaluated a single fraction of high-dose rate brachytherapy for low- and low-intermediate risk prostate cancers. After injection of 10 ml of macromolecular hyaluronic acid rectal spacer, needles were implanted under endorectal ultrasound guidance. After post-implant CT scan, a single fraction of 20 Gy was delivered to the prostate taking into account the dose constraints for CTV and organs at risk (urethra, rectum, anus). Oncological outcome (biochemical recurrence free survival), local (local recurrence free survival), and metastatic (metastatic recurrence free survival) relapses and disease-free (DFS), cause-specific (CSS) and overall (OS) survivals), toxicity profile (acute [d30, d90, d180] and late [> 6 months] genitourinary, gastrointestinal, and sexual toxicities) were investigated.
From March 2014 to October 2017, 35 patients were enrolled, of which 33 were evaluable. With a median age of 66 years (range 46 – 79), 25 (76%) and 8 (24%) patients were low-risk and favorable intermediate risk respectively. Median MRI prostatic volume was 41 cc (range 19 – 84). Median peak-urinary flow and post-void residual volume were 17 ml/s (range 8 – 25) and 19 cc (range 0 – 165), respectively, while 30 patients (91%) had an IIEF score between 16 and 25. Median CTV, D90% and V100% were 50 cc (range 29 – 92), 106% (range 104 – 109), and 98% (range 96 – 99), respectively. With a median follow-up of 72.8 months (range 64 – 86), 6 year-biochemical recurrence free survival, local recurrence free survival and metastatic recurrence free survival were 62% (45 – 85), 61% (44 – 85) and 93% (85 – 100), respectively while 6 year-DFS, CSS and OS were 54% (37 – 77), 100% and 89% (77 – 100):
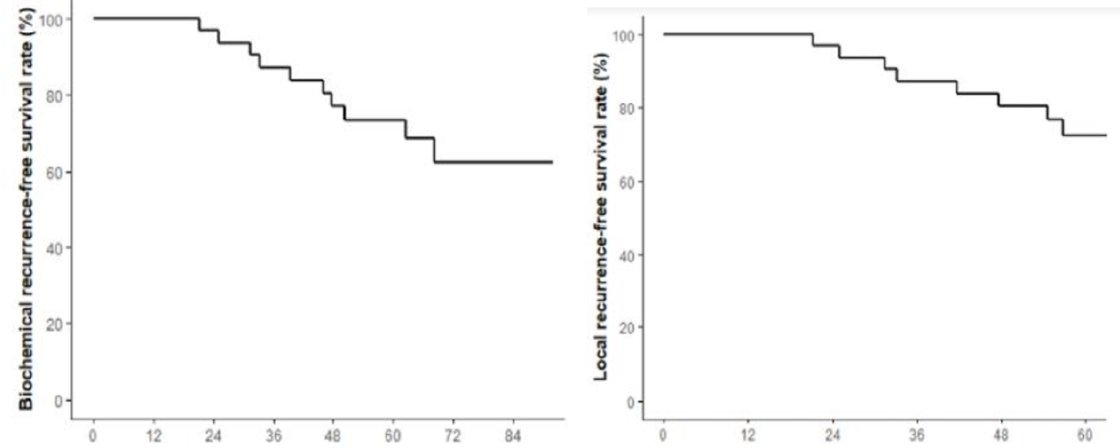
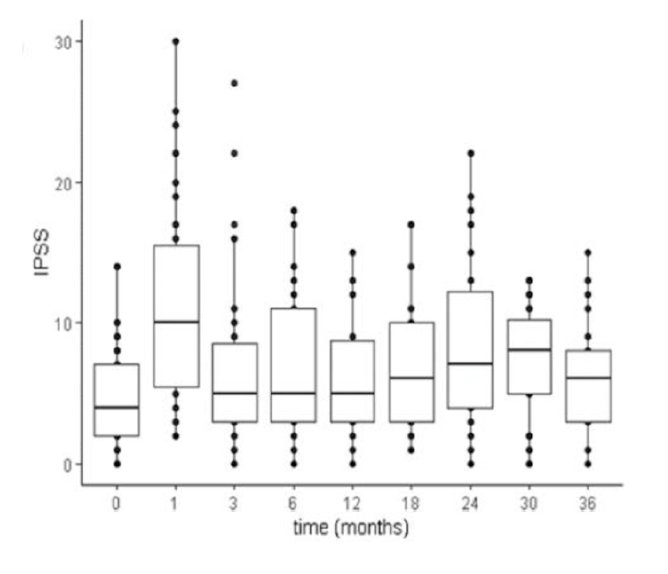
Acute genitourinary, gastrointestinal and sexual toxicities were observed in 27 patients (82%; 36 grade 1 and 8 grade 2), 5 patients (15%; 7 grade 1 and 3 grade 2) and 5 patients (15%; 3 grade 1 and 2 grade 2), respectively, and no acute grade 3 toxicity was observed. Late genitourinary, gastrointestinal and sexual toxicities were observed in 11 patients (33%; 18 grade 1), 4 patients (12%; 4 grade 1) and 7 patients (21%; 1 G1, 5 grade 2 and 1 grade 3), respectively. IPSS results are as follows:
Biochemical relapse was observed in 11 patients (33%; 7 low-risk and 4 favorable intermediate risk) with a median time interval between high-dose rate brachytherapy and biochemical relapse of 51 months (range 24 – 69). Among them, 9 patients (82%) presented a histologically proven isolated local recurrence and underwent salvage prostate re-irradiation delivering a median dose of 78 Gy (range 73 – 78; 2 Gy/fraction). With a median follow-up from salvage EBRT of 27 months (range 3 – 59), 2nd biochemical recurrence free survival rate was 100%. One patient presented a grade 3 genitourinary toxicity (hematuria) while no grade ≥3 gastrointestinal complication was observed.
Dr. Pujol concluded this presentation discussing the SiFEPI phase II trial assessing single fraction HDR brachytherapy for prostate cancer with the following concluding messages:
- Long-term results of the SiFEPI phase 2 prospective trial showed that a single fraction of 20 Gy (HDR brachytherapy) leads to a sub-optimal biochemical control for low-risk and low-intermediate risk prostate cancers
- The reported oncological outcome is in line with the previous series of HDR brachytherapy single fraction protocols
- However, late genitourinary and gastrointestinal toxicity profile is encouraging (especially compared to iodine implant series), leading to consideration of HDR brachytherapy as a safe irradiation technique
- Salvage EBRT appears feasible with an acceptable risk of complications
Presented by: Nina Pujol, MD, Antoine Lacassagne Cancer Center, Nice, France
Co-Authors: T. Pace-Loscos1, R. Schiappa2, M. Gautier1, M. E. Chand1, and J. M. Hannoun Levi3; 1Antoine Lacassagne Cancer Center, Nice, France, 2Antoine Lacassagne Cancer Center, University of Cote d’Azur, Nice, France, 3Department of Radiotherapy, Centre Antoine Lacassagne, University of Cote d'Azur, Nice, France
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2022 American Society of Radiation Oncology (ASTRO) Annual Hybrid Meeting, San Antonio, TX, Sat, Oct 22 – Wed, Oct 26, 2022.
References:
- Prada PJ, et al. High-dose-rate interstitial brachytherapy as monotherapy in one fraction for treatment of favorable stage prostate cancer: Toxicity and long-term biochemical results. Radiother Oncol. 2016;119(3):411-416.


