In this phase 2 trial (NCT02199197), patients with progressive mCRPC were treated with enzalutamide (160 mg daily) +/- radium-223 (standard dose of 55 kBq/kg IV Q4 weeks x 6), until disease progression or unacceptable toxicities. The primary objectives of change in bone markers and safety have been reported previously.2 Secondary objectives included a comparison of PSA progression free survival, overall survival, and long-term safety in all patients receiving enzalutamide plus radium-223 versus enzalutamide alone. A post hoc analysis included a comparison of PSA-PFS2 (defined as time from the start of protocol therapy to PSA progression on subsequent therapy or death whichever occurred earlier), time to subsequent/next therapy, and long-term safety.
Between July 2014 and November 2017, 49 patients were eligible and enrolled in this trial. The median follow-up was 22 months (range 3.2-71.5) and 35 patients received enzalutamide plus radium-223 and 12 patients received enzalutamide alone. Receipt of prior abiraterone was allowed and was balanced between two groups: 60% in the enzalutamide plus radium-223 group versus 64% in the enzalutamide alone group. In the final efficacy results, time to subsequent/next therapy and PSA-PFS2 were significantly improved in the enzalutamide plus radium-223 patients over enzalutamide alone patients:
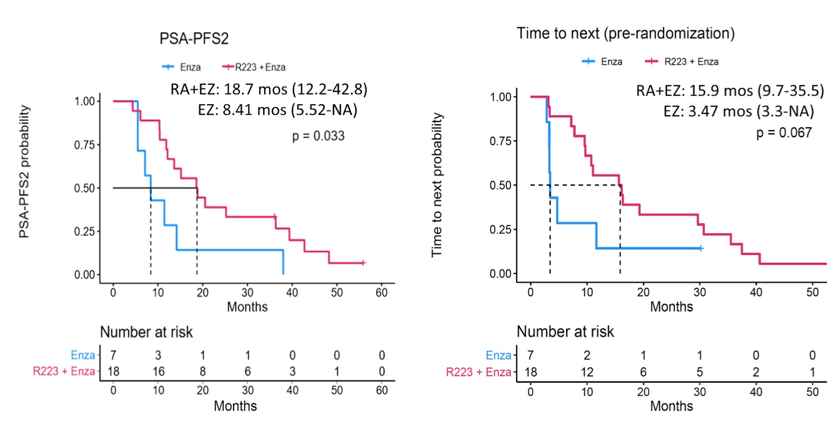
All other efficacy parameters (PSA-PFS, overall survival, and radiographic progression free survival) were numerically improved in enzalutamide plus radium-223 patients, but not statistically significant. With regards to the final safety results, none of the 12 enzalutamide alone patients had a fracture. There were two of 35 enzalutamide plus radium-223 patients found to have incidental grade 1 asymptomatic fracture at the site of bone metastasis on routine imaging, at 15 and 31 months, respectively after the last dose of radium-223, and did not require any intervention. No patients developed bone marrow disorders during the follow-up period.
Although a small, single institutional cohort, this study showed that enzalutamide plus radium-223 resulted in significant long-term clinical benefit over enzalutamide alone in patients with mCRPC without compromising safety.
Presented by: Adam Kessel, Huntsman Cancer Institute-University of Utah Health Care, Salt Lake City, UT
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia Twitter: @zklaassen_md during the 2021 ASCO Genitourinary Cancers Symposium (ASCO GU), February 11th to 13th, 2021
References:
- Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med 2013;369(3):213-223.
- Agarwal N, Nussenzveig R, Hahn AW, et al. Prospective evaluation of bone metabolic markers as surrogate markers of response to radium-223 therapy in metastatic castration-resistant prostate cancer. Clin Cancer Res. 2020 May 1;26(9):2104-2110.


