The first paper discussed by Dr. George was Motzer et al.'s “Nivolumab Versus Everolimus in Patients with Advanced Renal Cell Carcinoma: Updated Results with Long-Term Follow-Up of the Randomized, Open-Label, Phase 3 CheckMate 025 Trial” was published in Cancer.1 CheckMate 025 previously showed superior efficacy for nivolumab over everolimus in patients with advanced renal cell carcinoma along with improved safety and tolerability.2 Importantly, patients were previously treated with one or two antiangiogenic regimens. There were 821 patients randomized to nivolumab (n = 410) or everolimus (n = 411). In this extended analysis, with a minimum follow-up of 64 months (median 72 months), nivolumab maintained an overall survival benefit in comparison with everolimus (median 25.8 months, 95% confidence interval [CI] 22.2-29.8 versus 19.7 months, 95% CI 17.6-22.1; hazard ratio [HR] 0.73, 95% CI 0.62-0.85) with five-year overall survival probabilities of 26% and 18%, respectively:
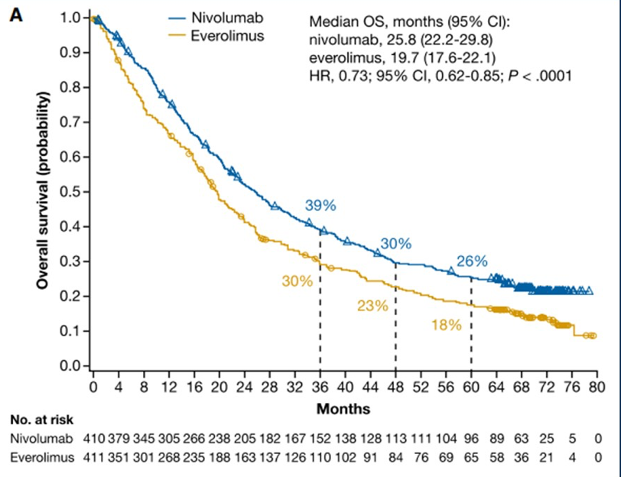
Objective response rate was higher with nivolumab (23% versus 4%; P < .001). Progression free survival also favored nivolumab (HR 0.84, 95% CI 0.72-0.99).
The second paper discussed by Dr. George was an extended follow-up of the CheckMate 214 study by Albiges et al. published in the ESMO Open: “Nivolumab plus ipilimumab versus sunitinib for first-line treatment of advanced renal cell carcinoma: extended 4-year follow-up of the phase III CheckMate 214 trial”.3 CheckMate 214 previously showed a survival benefit for first-line nivolumab plus ipilimumab for intermediate/poor-risk metastatic renal cell carcinoma (mRCC) versus sunitinib.4 In this trial, patients were randomized to nivolumab (3 mg/kg) plus ipilimumab (1 mg/kg) every three weeks ×4 doses, followed by nivolumab (3 mg/kg) every two weeks versus sunitinib (50 mg) once per day ×4 weeks (6-week cycle). There were 1,096 patients randomized and after four years of minimum follow-up, overall survival remained superior with nivolumab plus ipilimumab versus sunitinib in the intention to treat population (HR 0.69, 95% CI 0.59 -0.81) and in intermediate/poor risk patients (HR 0.65, 95% CI 0.54-0.78). Four-year progression-free survival probabilities were 31.0% versus 17.3% in the intention to treat population and 32.7% vs. 12.3% for intermediate/poor-risk patients, with nivolumab plus ipilimumab versus sunitinib. Objective response rates also remained higher with nivolumab plus ipilimumab versus sunitinib in the intention to treat population (39.1% vs. 32.4%) and for patients with intermediate/poor risk (41.9% vs. 26.8%) patients.
Dr. George notes that sarcomatoid dedifferentiation is consistent with an aggressive cancer phenotype that results in poor outcomes, and that median overall survival in the tyrosine kinase inhibitor (TKI)/mTOR era is only approximately 11 months. The third paper discussed by Dr. George was by Rini et al.: “Atezolizumab plus bevacizumab versus sunitinib for patients with untreated metastatic renal cell carcinoma and sarcomatoid features: A Prespecified subgroup analysis of the IMmotion151 clinical trial” published in European Urology.5 The objective of this study was to perform a prespecified analysis of the Phase III IMmotion151 trial in previously untreated patients with advanced or mRCC to assess the effectiveness of atezolizumab plus bevacizumab versus sunitinib in a subgroup of patients with sarcomatoid features. Patients whose tumors had any component of sarcomatoid features were included and received atezolizumab plus bevacizumab (n = 68) or sunitinib (n = 74). The median progression-free survival was significantly longer in the group receiving atezolizumab + bevacizumab overall (8.3 versus 5.3 months; HR 0.52, 95% CI 0.34-0.79) and in the subset of patients with PD-L1-positive tumors (8.6 versus 5.6 months; HR 0.45, 95% CI 0.26-0.77):
More patients receiving atezolizumab plus bevacizumab achieved an objective response (49% vs. 14%), including complete responses (10% vs. 3%), and reported greater symptom improvements versus sunitinib.
Finally, Dr. George discussed “Molecular Subsets in Renal Cancer Determine Outcome and Angiogenesis Blockade” by Motzer et al. published in Cancer Cell.6 This study included 823 tumors from advanced RCC patients for which molecular subsets associated with differential clinical outcomes to angiogenesis blockade alone or with a checkpoint inhibitor were identified. Unsupervised transcriptomic analysis revealed seven molecular subsets with distinct angiogenesis, immune, cell-cycle, metabolism, and stromal programs. Somatic mutations in PBRM1 and KDM5C were associated with high angiogenesis and AMPK/fatty acid oxidation gene expression, while CDKN2A/B and TP53 alterations were associated with increased cell-cycle and anabolic metabolism. Finally, sarcomatoid tumors exhibited a lower prevalence of PBRM1 mutations and angiogenesis markers, frequent CDKN2A/B alterations, and increased PD-L1 expression.
Dr. George provided the following take-home messages from his presentation discussing highlights of renal cell carcinoma papers published in 2020 from a medical oncology perspective:
- Single-agent checkpoint inhibitor nivolumab is safe and efficacious in the five-year updated data. Roughly 20% of responders did not need a subsequent treatment and were alive at five years
- A four-year update of the CheckMate 214 trial is consistent with nivolumab plus ipilimumab with long and durable responses. Roughly 50% of responders were having ongoing response with or without treatment
- Outcomes of sarcomatoid RCC have been revolutionized by immunotherapy combinations
- Novel pathways/biomarker development promises a bright future
Written by: Zachary Klaassen, MD, MSc, Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, Augusta, Georgia, Twitter: @zklaassen_md during the 2021 American Society of Clinical Oncology Genitourinary Cancers Symposium (#GU21), February 11th-February 13th, 2021
References:
1. Motzer, Robert J., Bernard Escudier, Saby George, Hans J. Hammers, Sandhya Srinivas, Scott S. Tykodi, Jeffrey A. Sosman et al. "Nivolumab versus everolimus in patients with advanced renal cell carcinoma: Updated results with long‐term follow‐up of the randomized, open‐label, phase 3 CheckMate 025 trial." Cancer 126, no. 18 (2020): 4156-4167.
2. Motzer, Robert J., Bernard Escudier, David F. McDermott, Saby George, Hans J. Hammers, Sandhya Srinivas, Scott S. Tykodi et al. "Nivolumab versus everolimus in advanced renal-cell carcinoma." New England Journal of Medicine 373, no. 19 (2015): 1803-1813.
3. Albiges, Laurence, Nizar M. Tannir, Mauricio Burotto, David McDermott, Elizabeth R. Plimack, Philippe Barthélémy, Camillo Porta et al. "Nivolumab plus ipilimumab versus sunitinib for first-line treatment of advanced renal cell carcinoma: extended 4-year follow-up of the phase III CheckMate 214 trial." ESMO open 5, no. 6 (2020): e001079.
4. Motzer, Robert J., Nizar M. Tannir, David F. McDermott, Osvaldo Arén Frontera, Bohuslav Melichar, Toni K. Choueiri, Elizabeth R. Plimack et al. "Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma." New England Journal of Medicine (2018).
5. Rini, Brian I., Robert J. Motzer, Thomas Powles, David F. McDermott, Bernard Escudier, Frede Donskov, Robert Hawkins et al. "Atezolizumab plus bevacizumab versus sunitinib for patients with untreated metastatic renal cell carcinoma and sarcomatoid features: a prespecified subgroup analysis of the IMmotion151 clinical trial." European Urology (2020).
6. Motzer, Robert J., Romain Banchereau, Habib Hamidi, Thomas Powles, David McDermott, Michael B. Atkins, Bernard Escudier et al. "Molecular subsets in renal cancer determine outcome to checkpoint and angiogenesis blockade." Cancer Cell 38, no. 6 (2020): 803-817.


