Patients with up to two lines of prior systemic therapy with one to five oligometastases from clear cell RCC were eligible. A single fraction of 20 Gy stereotactic ablative body radiotherapy to all metastatic sites was given (or 10 fractions of 3 Gy of conventional radiotherapy if stereotactic ablative body radiotherapy was not feasible), followed by pembrolizumab 200 mg administered Q3W for eight cycles. The primary objective was safety (Common Terminology Criteria for Adverse Events [CTCAE] v4.03), with secondary key objectives of efficacy (Response Evaluation Criteria in Solid Tumours [RECIST] 1.1) by disease control rate, defined as complete response, partial response, or stable disease for at least six months, objective response rate, progression-free survival, and overall survival.
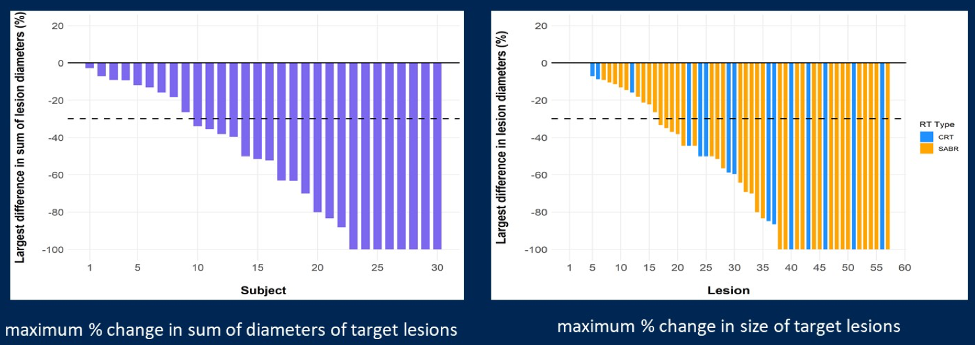
Thirty patients were enrolled and received protocol treatment. The median age was 62 (range 47-80) years, 23 patients (77%) were male, and 23 patients (77%) were treatment-naïve, one patient (3%) had prior interleukin-2 therapy, and six patients (20%) had a prior tyrosine kinase inhibitor; nine patients (30%) had prior metastasectomy. Eighty-three oligometastases were treated (median of three per patient), of which 64 (77%) received stereotactic ablative body radiotherapy, and 19 (23%) received conventional radiotherapy. There were eight adrenal metastases, 11 bone, 43 lung, 12 lymph node, and nine soft tissue metastases irradiated. Four patients (13%, 95% confidence interval [CI] 4-31%) had one or more grade 3 treatment-related adverse events, including pneumonitis (n=2), dyspnea (n=1) and elevated ALP/ALT (n=1). There were no grade 4 or 5 adverse events. All eight cycles of pembrolizumab were completed by 24 (80%) patients. Over a median follow-up of 2.3 years, the disease control rate was 83% (95% CI 65-94%). The complete response rate for stereotactic ablative body radiotherapy was 40%, partial response rate was 23%, and stable disease rate was 23%. As follows are the results for maximum percent change in the sum of diameters of target lesions and maximum percent change in the size of target lesions:
The median progression-free survival was 15.6 months, and the median overall survival and median duration of response (complete or partial response) was not reached: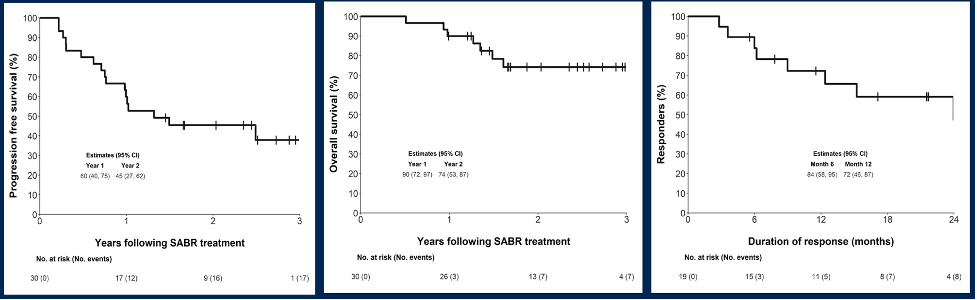
Estimated one and two-year overall survival rates were 90% (95% CI 72-97%) and 74% (95% CI 53-87%), respectively, while progression-free survival was 60% (95% CI 40-75%) and 45% (95% CI 27-62%), respectively. Freedom from local progression at two years was 92% (95% CI 80-97%).
Dr. Siva concluded this presentation of the RAPPORT trial with the following take-home messages:
- The combination of stereotactic ablative body radiotherapy and a short course of pembrolizumab in oligometastatic renal cell carcinoma is well tolerated with excellent local control
- The observed progression-free survival of 15.6 months and objective response rate of 63% compares favorably to historic KEYNOTE-427 pembrolizumab monotherapy results of 7.1% months and objective response rate of 34%
- Durable responses and encouraging progression-free survival were observed with this approach, which warrants further investigation
Written by: Zachary Klaassen, MD, MSc, Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, Augusta, Georgia, Twitter: @zklaassen_md during the 2021 American Society of Clinical Oncology Genitourinary Cancers Symposium (#GU21), February 11th-February 13th, 2021


