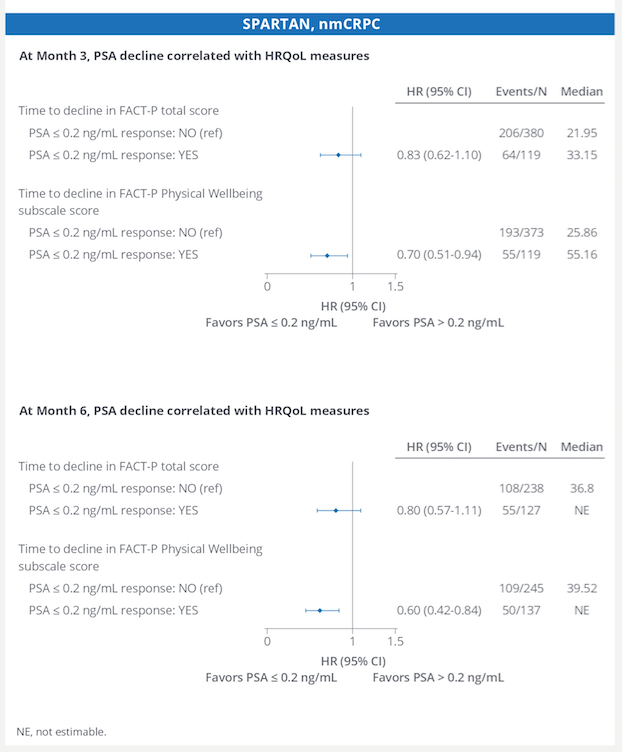
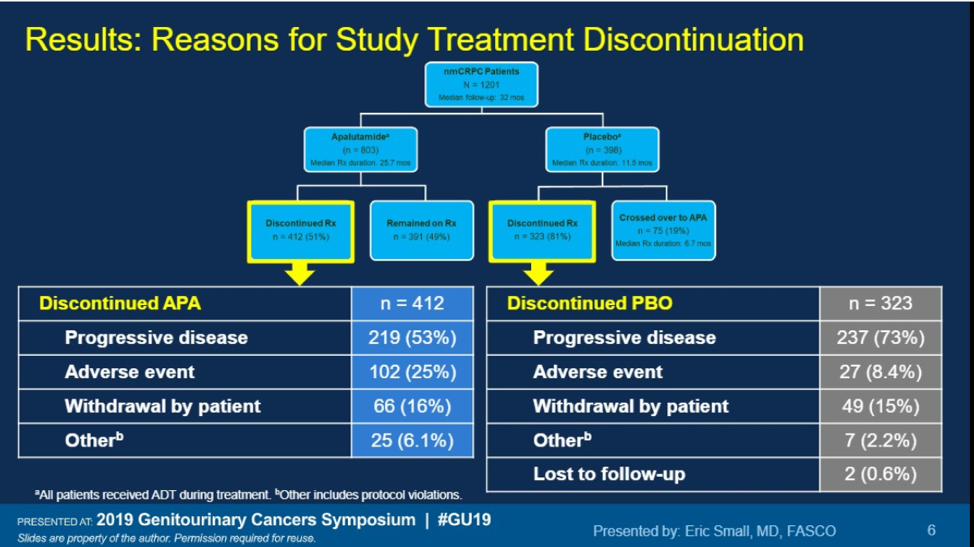
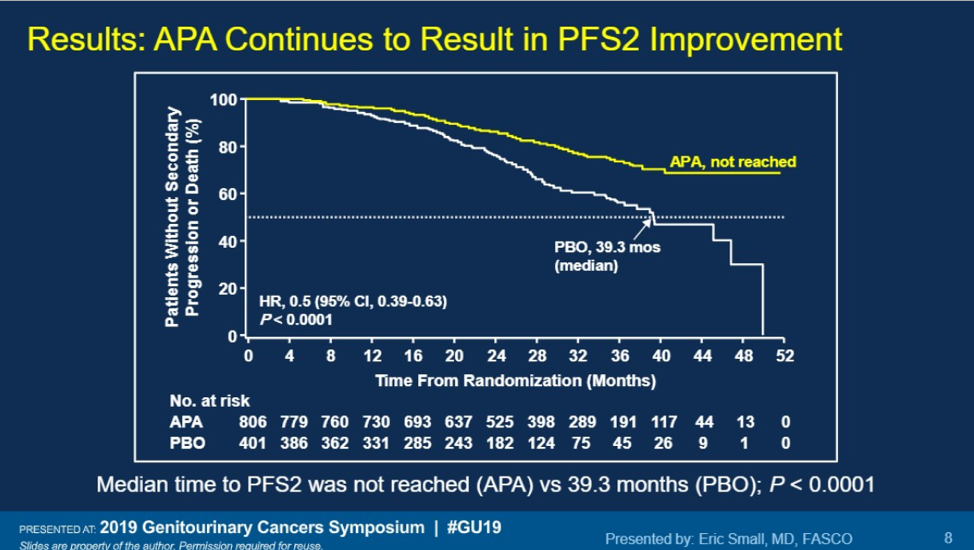
Apalutamide continues to show benefit for patients with nmCRPC, not only for increasing metastasis-free survival, but also for increasing PFS2, with a favorable side effect profile. With data coming out this GU ASCO on darolutamide, there will soon be three options (enzalutamide, apalutamide, darolutamide) for patients with nmCRPC and patient factors will be important to consider which is the best-personalized option.
Presented by: Eric J. Small, MD, FASCO, UCSF Helen Diller Family Comprehensive Cancer Care Center
Discussant: Nicholas J. van As, MBBCH, MRCP, FRCR, MD(res), Institute of Cancer Research and The Royal Marsden NHS Foundation Trust
Written By: Jason Zhu, MD. Fellow, Division of Hematology and Oncology, Duke University, Twitter: @TheRealJasonZhu at the 2019 American Society of Clinical Oncology Genitourinary Cancers Symposium, (ASCO GU) #GU19, February 14-16, 2019 - San Francisco, CA
References:


