Breaking News: Darolutamide Receives FDA Approval for the Treatment of Non-metastatic Castration-resistant Prostate Cancer
San Francisco, CA (UroToday.com) The use of androgen-axis targeted agents, specifically enzalutamide and abiraterone, have drastically changed the landscape of advanced prostate cancer management. Just last year, at GU ASCO 2018, two landmark trials were presented – SPARTAN and PROSPER - which were conducted in nonmetastatic CRPC (nmCRPC) patients.1,2 PROSPER specifically assesses enzalutamide in the M0 CRPC setting, while SPARTAN focused on apalutamide-ARN-509 (APA). Apalutamide is a next-generation competitive inhibitor of the androgen receptor under development for the treatment of patients with prostate cancer but perhaps with greater potency and reduced CNS effects. Both demonstrated ~2 year metastases-free survival benefit compared to ADT alone and there was early evidence of OS benefit.
Darolutamide Unique Properties
Yet, further agents are being developed in the ARAT axis. One such agent is darolutamide, an agent related to enzalutamide and apalutamide – however, darolutamide is a structurally unique androgen receptor (AR) antagonist, with inherently different pharmacokinetics. For one, it does not cross the blood-brain barrier, which could result in less CNS toxicity. It also had been demonstrated to have a high affinity for AR, low affinity for GABA receptors, and therefore a lower potential for drug-drug interactions. Phase 1/2 trial data from the ARADES and ARAFOR trials demonstrated significant PSA response (>50% decline in 65-83% of men) with minimal drug toxicity in men with chemotherapy naïve mCRPC.3,4
ARAMIS Trial Design
In this presentation, Dr. Fizazi and colleagues present the efficacy and safety data of ARAMIS study. This was a double-blind, placebo-controlled phase III trial randomized controlled trial in which nmCRPC patients were randomized in a 2:1 ratio to receive either darolutamide 600 mg (two 300 mg tablets) twice-daily or placebo, while continuing androgen deprivation therapy (ADT). Patients were stratified by prostate-specific antigen doubling time (≤6 months or >6 months) and use of osteoclast-targeted therapy, similar to the PROSPER and SPARTAN studies.
Inclusion criteria were men with: PSA >= 2 ng/mL, ECOG 0-1, and PSADT <= 10 months. The full study protocol can be seen below:

The primary endpoint was metastasis-free survival (MFS), with independent central review of radiographic imaging every 16 weeks. MFS was defined as distant metastases OR death from any cause. Importantly, if independent central review identified baseline metastases, these remained a “baseline” event and were not considered for progression.
Secondary endpoints include overall survival (OS), times to pain progression (assessed by Brief Pain Inventory), first cytotoxic chemotherapy and first symptomatic skeletal event, as well as safety profile.
In total, 1,509 patients were randomized in the study – 955 men to darolutamide and 554 men to placebo. Demographics of the two groups are seen and summarized below:
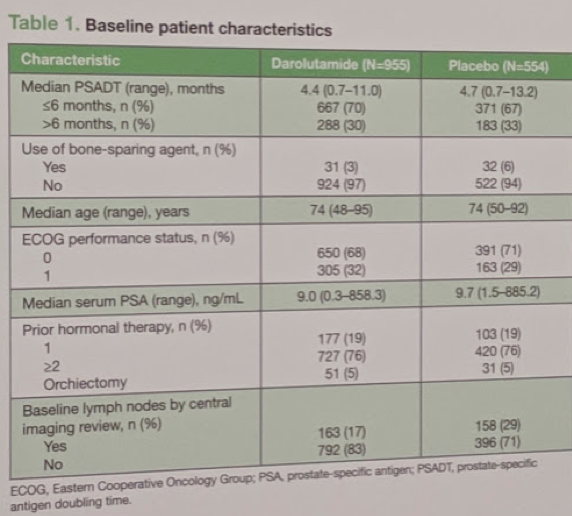
Median PSA doubling time was fast, ~4.5 months. There was minimal (<5%) bone-sparing agents utilized in either group.
At the time of data cutoff, 64% of the darolutamide and 36% of the placebo arm patients were still receiving the assigned treatment. It should be noted that most of these men (~70%) had PSADT <=6 months and many had seen >= 2 prior hormonal therapies.
Oncologic Outcomes
In terms of oncologic outcomes, the primary outcome was MFS. Median MFS was 40.4 months with darolutamide vs 18.4 months with placebo (hazard ratio [HR] 0.41; 95% confidence interval [CI] 0.34–0.50; 2-sided p<0.0001). This represents a 59% reduction in metastases or death.

Subgroup Analyses
On subgroup analyses, this benefit was seen across all major subgroups, as seen in the figure below (only exception was Hispanics and other races, likely due to small sample size):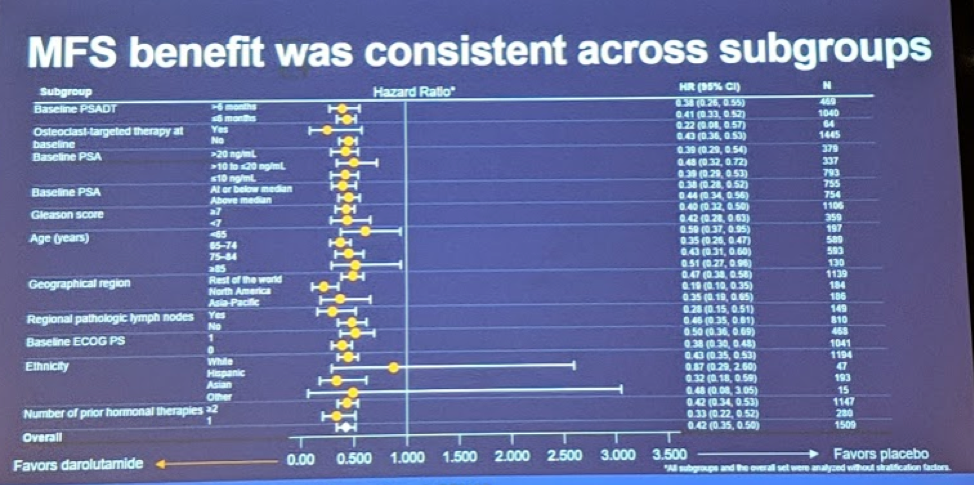
Overall survival (OS) showed a trend in favor of darolutamide (Median not reached for either arm, HR 0.71, 95% CI 0.50–0.99, 2-sided p=0.045), as did time to pain progression (HR 0.65; 95% CI 0.53–0.79; 2-sided p<0.0001). These results are remarkably similar to apalutamide and enzalutamide. However, OS data is still immature and the final analysis will be presented in the future.
Other secondary and exploratory efficacy endpoints also favored darolutamide over placebo.
- - Progression-free Survival: 62% reduced risk of local progression, distant metastases or death (36.8 months vs. 14.8 months, HR 0.38, p < 0.0001)
- - Time to first cytotoxic chemotherapy: HR 0.43, p < 0.001
- - Time to SSRE: HR 0.43, p = 0.011
Adverse Events
Incedences of treatment-emergent adverse events (AEs) with ≥5% frequency or grade 3–5 were comparable between darolutamide and placebo arms. Only fatigue occurred in >10% of men. Total Grade 3-5 AE’s were similar between the two groups: 24.7% in the darolutamide arm vs. 19.5% in the placebo arm.
Discontinuation rates due to AEs were similar between the 2 groups: 8.9% with darolutamide and 8.7% with placebo.
An interesting analysis was their assessment of AE’s (grouped into categories) often seen with other AR inhibitors - including fracture, falls, seizures, weight decrease, hypertension, and cognitive disorder - showed minimal or no difference in incidence between study arms. This is seen in the table below:

Based on this, Dr. Fizazi noted that darotulamide was well-tolerated, had similar efficacy to established agents in this disease space (compared to ADT alone), and had minimal adverse events.5
Finally, as a comparison, I have compared the study to SPARTAN and PROSPER below:
Summary of SPARTAN, PROSPER and ARAMIS Studies

Presented by: Karim Fizazi, MD, Ph.D., medical oncologist, Head of the Department of Cancer Medicine at the Institut Gustave Roussy, Villejuif, France and Professor in Oncology at the University of Paris
Written by: Thenappan Chandrasekar, MD (Clinical Instructor, Thomas Jefferson University) (twitter: @tchandra_uromd, @JEFFUrology) at the 2019 American Society of Clinical Oncology Genitourinary Cancers Symposium, (ASCO GU) #GU19, February 14-16, 2019 - San Francisco, CA
References:
- Hussain M, Fizazi K, Saad F, Rathenborg P, Shore N, Ferreira U, et al. Enzalutamide in men with nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2018;378(26):2465–74.
- Smith MR, Saad F, Chowdhury S, Oudard S, Hadaschik BA, Graff JN, et al. Apalutamide treatment and metastasis-free survival in prostate cancer. N Engl J Med. 2018;378(15).
- Fizazi K, Massard C, Bono P, Jones R, Kataja V, James N, Garcia JA, Protheroe A, Tammela TL, Elliott T, Mattila L, Aspegren J, Vuorela A, Langmuir P, Mustonen M; ARADES study group. Activity and safety of ODM-201 in patients with progressive metastatic castration-resistant prostate cancer (ARADES): an open-label phase 1 dose-escalation and randomised phase 2 dose expansion trial. Lancet Oncol. 2014 Aug;15(9):975-85. doi: 10.1016/S1470-2045(14)70240-2. Epub 2014 Jun 25.
- Massard C, Penttinen HM, Vjaters E, Bono P, Lietuvietis V, Tammela TL, Vuorela A, Nykänen P, Pohjanjousi P, Snapir A, Fizazi K. Pharmacokinetics, Antitumor Activity, and Safety of ODM-201 in Patients with Chemotherapy-naive Metastatic Castration-resistant Prostate Cancer: An Open-label Phase 1 Study. Eur Urol. 2016 May;69(5):834-40. doi: 10.1016/j.eururo.2015.09.046. Epub 2015 Oct 17.
- Fizazi, K., Shore, N., Tammela, T., Ulys, A., Vjaters, E., & Polyakov, S. et al. (2019). Darolutamide in Nonmetastatic, Castration-Resistant Prostate Cancer. New England Journal Of Medicine. doi: 10.1056/nejmoa1815671


