
This is a single arm, phase II study for patients with BCG refractory non-muscle invasive bladder cancer (NMIBC). Patients had histologically confirmed high-risk disease, defined as carcinoma in situ, T1 tumor, and/or high-grade Ta disease. The definition of BCG unresponsiveness required that patients first had adequate BCG therapy (patients with ≥5 doses of BCG of an induction course plus at least 2 doses of maintenance therapy or 2 doses of second induction course) and they were refractory/relapsing (see below).
This abstract describes the outcomes of patients in cohort A, those with carcinoma in situ (CIS) with or without papillary disease (high-grade Ta or T1). In terms of treatment, patients received pembrolizumab 200 mg every 3 weeks for 2 years or until recurrence, progression, or unacceptable toxicity.
A total of 102 patients were enrolled. The majority of patients were men (83%). These patients had a median of 12 prior BCG instillations.

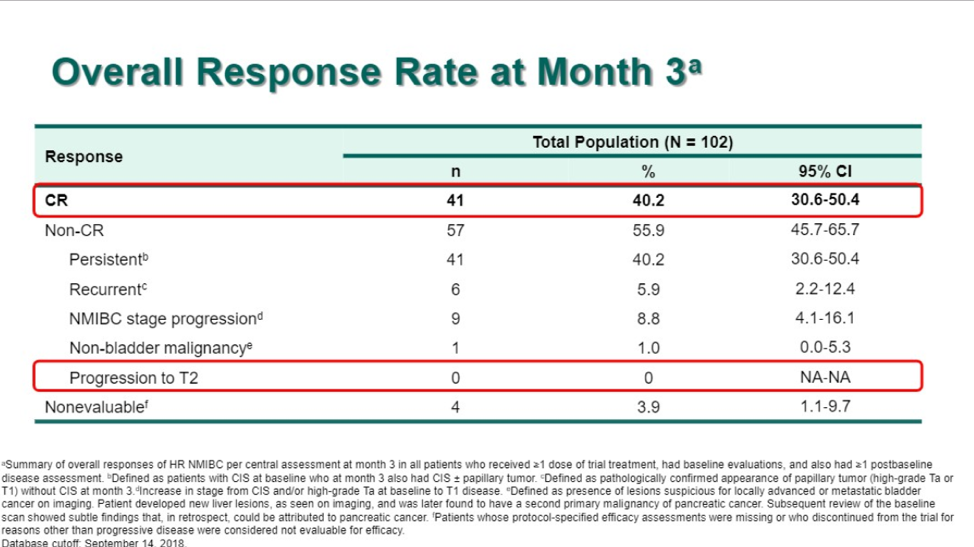
After a median follow up of 15.8 months, the complete response rate was 40.2%, with a median CR duration of 12.7 months. 36.6% of patients developed recurrent NMIBC after having a complete response and no patients progressed to muscle-invasive disease/metastatic disease.
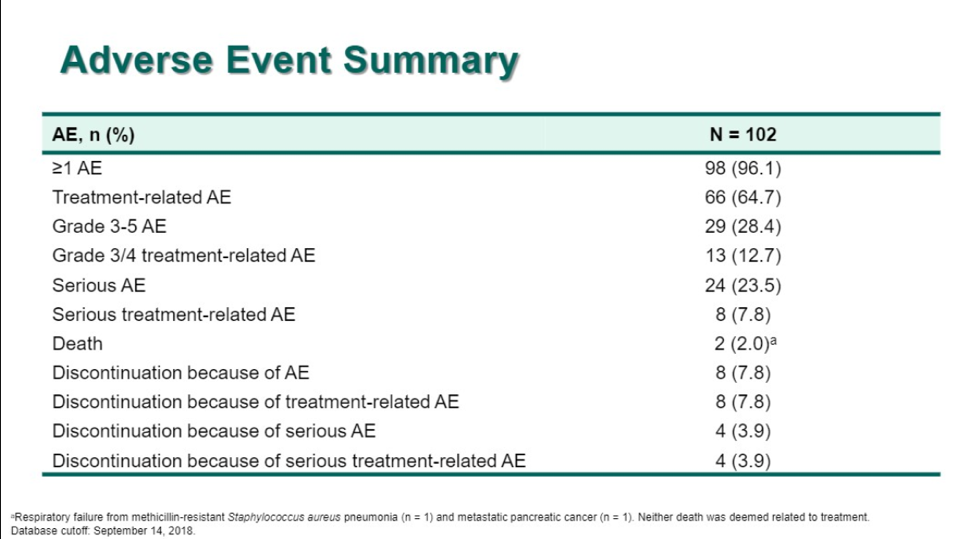
In terms of safety, 28% of patients developed grade 3/4 treatment-related events. Grade 3/4 immune-mediated adverse events occurred in 3% of patients and there was 1 treatment-related death secondary to immune-mediated colitis (which was re-classified to cohort B at the time of this presentation).
Pembrolizumab may be effective at inducing a complete response in up to 40% of patients with BCG refractory NMIBC. Very few patients had grade 3/4 immune-related adverse events. A phase 3 study evaluating pembrolizumab in high risk non-muscle invasive bladder cancer that is persistent after BCG induction (KEYNOTE 676) is underway
Clinical Trial Information: NCT02625961
Presented by: Arjun V. Balar, MD, NYU Langone Perlmutter Cancer Center, New York, New York
Written by: Jason Zhu, MD. Fellow, Division of Hematology and Oncology, Duke University @TheRealJasonZhu at the 2019 American Society of Clinical Oncology Genitourinary Cancers Symposium, (ASCO GU) #GU19, February 14-16, 2019 - San Francisco, CA
References:
- Sylvester RJ, van der Meijden AP, Oosterlinck W, et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. European Urology 2006;49:466-77.
- Babjuk M, Böhle A, Burger M, et al. EAU guidelines on non–muscle-invasive urothelial carcinoma of the bladder: update 2016. European Urology 2017;71:447-61.
- Chang SS, Boorjian SA, Chou R, et al. Diagnosis and treatment of non-muscle invasive bladder cancer: AUA/SUO guideline. The Journal of Urology 2016;196:1021-9.
- Kassouf W, Traboulsi SL, Kulkarni GS, et al. CUA guidelines on the management of non-muscle invasive bladder cancer. Canadian Urological Association Journal 2015;9:E690.
- Davis JW, Sheth SI, Doviak MJ, Schellhammer PF. Superficial bladder carcinoma treated with bacillus Calmette-Guerin: progression-free and disease-specific survival with minimum 10-year follow up. The Journal of Urology 2002;167:494-501.
- Necchi A, Briganti A, Bianchi M, et al. Preoperative pembrolizumab (pembro) before radical cystectomy (RC) for muscle-invasive urothelial bladder carcinoma (MIUC): Interim clinical and biomarker findings from the phase 2 PURE-01 study. American Society of Clinical Oncology; 2018.


