(UroToday.com) The 2023 ASCO annual meeting included a prostate cancer session, featuring a presentation by Dr. Syed Arsalan Ahmed Naqvi discussing a meta-analysis of the role of volume of disease for treatment selection in patients with metastatic castration sensitive prostate cancer (mCSPC).
Previous evidence suggests that there may be no additional benefit of triplet therapy in low volume disease based on limited data. Importantly, the ENZAMET and ARASENS trials have recently updated data for triplet therapy by volume of disease.1,2 Therefore, Dr. Naqvi and colleagues investigated the efficacy of triplet therapy as compared to docetaxel and androgen pathway inhibitor doublets by volume of disease using the most up to date results from the ARASENS trial.
Phase III randomized controlled trials assessing treatment intensification with androgen pathway inhibitor, and/or docetaxel in patients with mCSPC were included. Precomputed hazard ratios (HR) with 95% confidence intervals (CI) for OS were pooled using an inverse-variance approach, and a DerSimonian-Laird random-effects meta-analysis was conducted to assess the efficacy of triplet therapy compared to docetaxel doublet therapy. P-value of interaction was computed to assess difference between high and low volume disease subgroups (with a Pint <0.1 indicating statistical significance). Additionally, mixed treatment comparisons were computed using network meta-analysis to assess the comparative effectiveness of triplet therapy compared to androgen pathway inhibitor doublets by volume of disease.
Pairwise meta-analysis included a total of 3 randomized trials (ARASENS, PEACE-1, ENZAMET) with 2,518 patients (high volume:3 1,820; low volume: 698) as shown in the following tables:
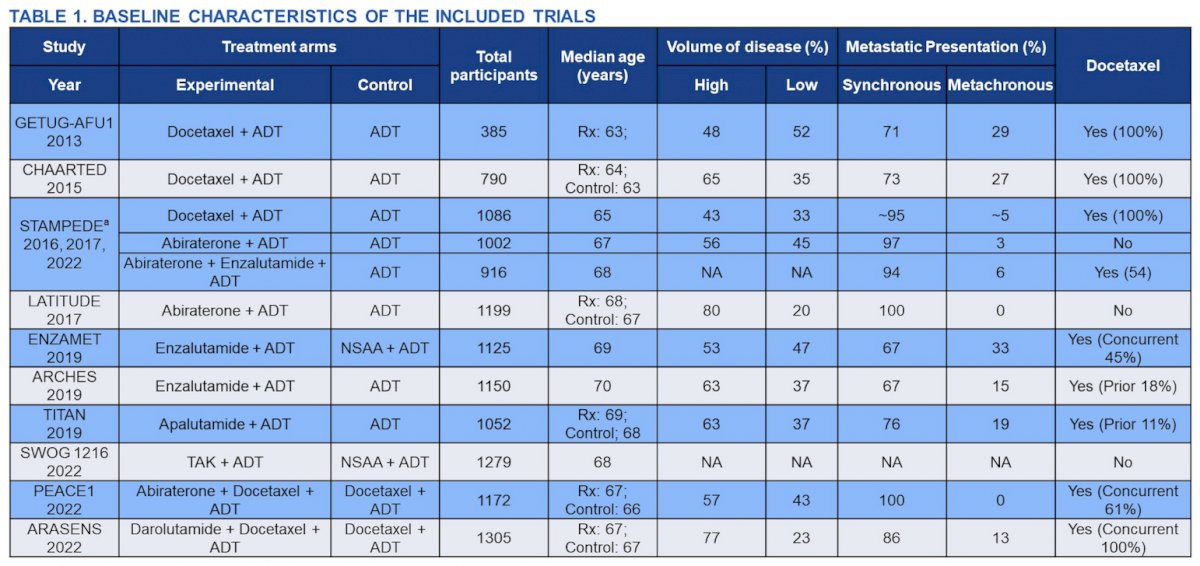
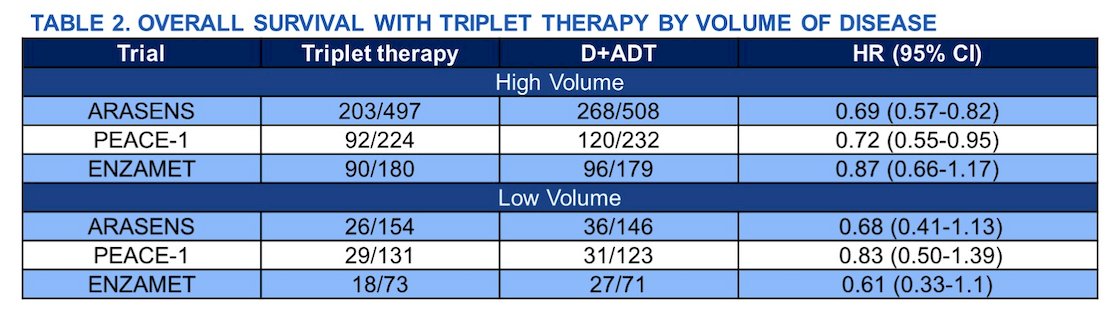
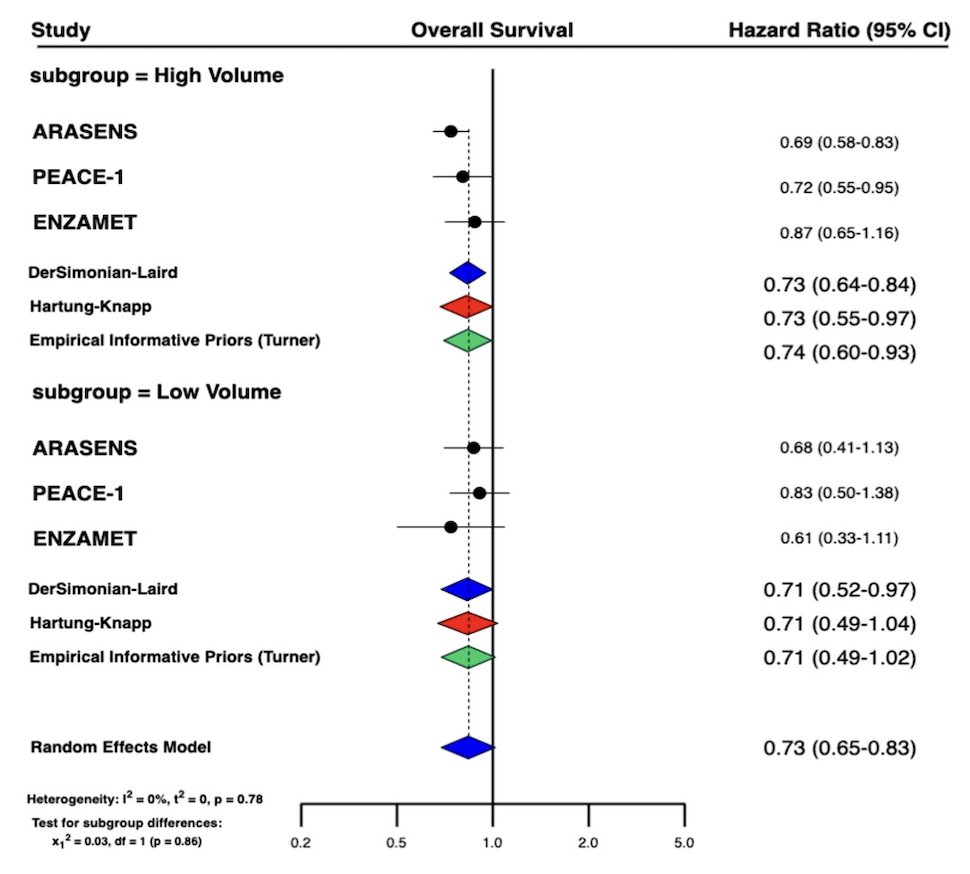
In patients with high volume disease, 385 (43%) and 484 (53%) deaths were observed with triplet therapy, and docetaxel doublet, respectively. Triplet therapy significantly improved OS as compared to docetaxel doublet in high volume disease (HR: 0.73; 95% CI: 0.64-0.84). In patients with low volume disease, 73 (20%) and 94 (28%) deaths were observed with triplet therapy, and docetaxel doublet, respectively. Triplet therapy also showed significantly improved OS as compared to docetaxel doublet in low volume (HR: 0.71; 95% CI: 0.52-0.97). There was no statistically significant interaction by volume of disease for triplet therapy vs. docetaxel doublet (Pint: 0.86):
Network meta-analysis including 10 clinical trials and over 11,500 patients updated as of February 13, 2023 showed that in high volume mCSPC, triplet therapy was ranked as potentially the most efficacious treatment option and may improve OS compared to androgen pathway inhibitor doublet therapy (HR: 0.83; 95% CI: 0.66-1.03). In low volume mCSPC, androgen pathway inhibitor doublet therapy was ranked as potentially the most efficacious treatment followed by triplet therapy. There was no significant difference between triplet therapy and androgen pathway inhibitor doublet therapy (HR: 1.12; 95% CI: 0.74-1.72). As follows is the League table for overall survival: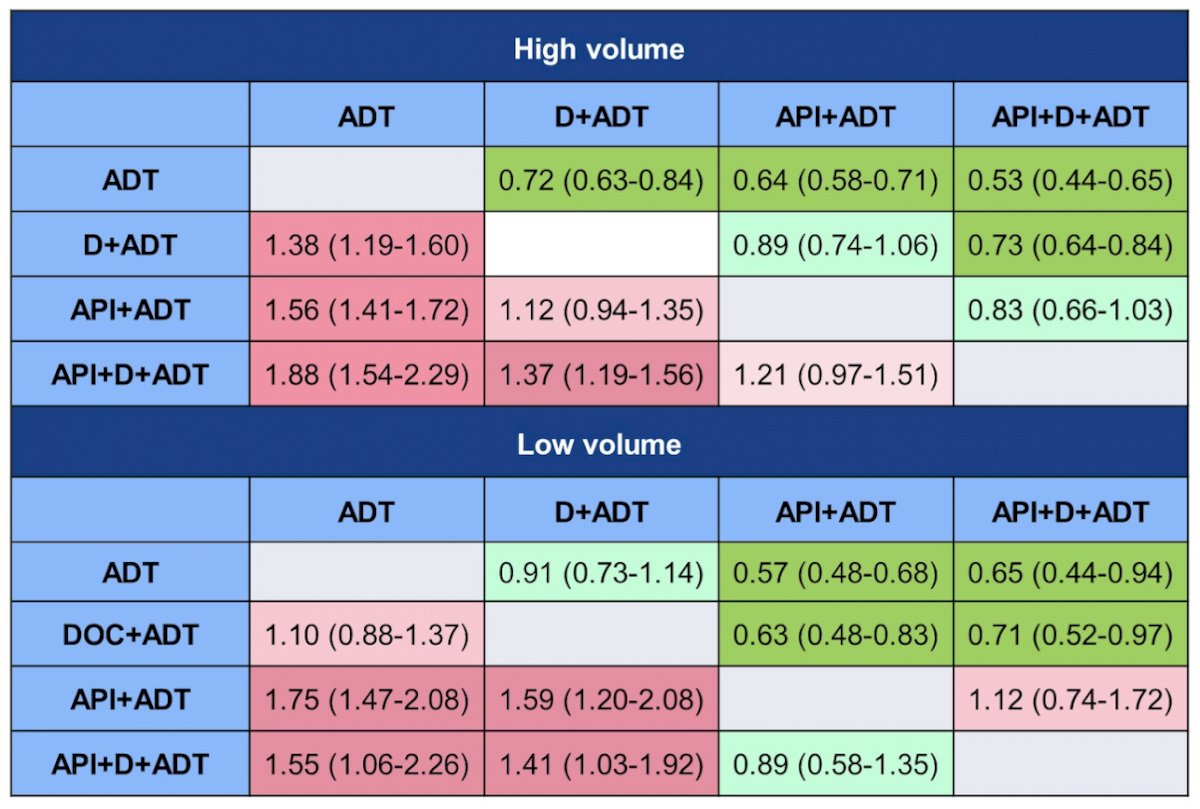
Finally, the following figure shows the rank plot for overall survival, showing that ADT + docetaxel + androgen pathway inhibitor is favored for high volume disease, and ADT + androgen pathway inhibitor is favored for low volume disease: 
Dr. Naqvi concluded his presentation discussing a meta-analysis of the role of volume of disease for treatment selection in patients with mCSPC with the following take-home points:
- The results indicate that triplet therapy may be preferred in mCSPC patients with high volume disease
- On the other hand, androgen pathway inhibitor doublet therapy may be preferred in mCSPC patients with low volume disease
- These findings suggest that the choice of treatment should be tailored to the individual patient’s disease volume
Presented by: Syed Arsalan Ahmed Naqvi, MBBS, Mayo Clinic, Rochester, MN
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2023 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, June 2 – Tues, June 6, 2023.
References:
- Sweeney CJ, Martin AJ, Stockler MR, et al. Testosterone suppression plus enzalutamide versus testosterone suppression plus standard antiandrogen therapy for metastatic hormone-sensitive prostate cancer (ENZAMET): An international, open-label, randomized, phase 3 trial. Lancet Oncol. 2023 Apr;24(4):323-334.
- Hussain M, Tombal B, Saad F, et al. Darolutamide plus androgen-deprivation therapy and docetaxel in metastatic hormone-sensitive prostate cancer by disease volume and risk subgroups in the phase III ARASENS trial. J Clin Oncol. 2023 Feb 16 [Epub ahead of print].
- Fizazi K, Foulon S, Carles J, Roubaud G, et al. Abiraterone plus prednisone added to androgen deprivation therapy and docetaxel in de novo metastatic castration-sensitive prostate cancer (PEACE-1): A multicentre, open-label, randomized, phase 3 study with a 2 x 2 factorial design. Lancet. 2022 Apr 30;399(10336):1695-1707.


