(UroToday.com) Norman Sharpless, MD, the director of the National Cancer Institute (NCI), discussed the state of cancer research during the global pandemic of COVID-19 at the virtual ASCO 2020 annual meeting.
The impacts of the COVID-19 pandemic on cancer care and research have been quite significant, according to Dr. Sharpless. These have resulted in:
- Delayed diagnosis and upstaging of patients with cancer
- A substantial decrease in the screening of cancer
- Reduced or non-standard care
- A significant decrease in participation in clinical trials and drug discovery activities
- The increased cost of clinical trials
- Slowing of basic cancer research since many cancer research labs were closed
Dr. Sharpless continued his talk by displaying a graph of patient accrual to clinical trials by week starting with January 5, 2020, and continuing through May 10, 2020 (Figure 1). It clear that there is a significant drop starting at the end of March and continuing through May 10, 2020. 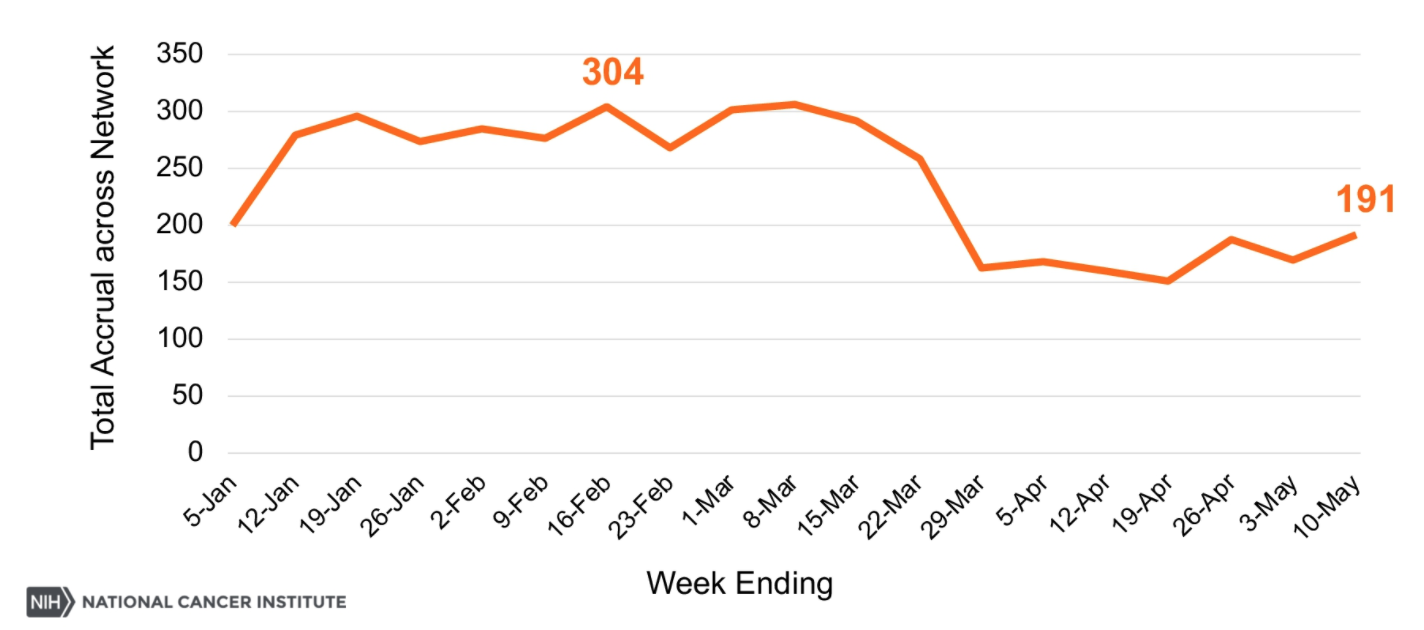
Figure 1. Accrual in cancer clinical trials by week
Dr. Sharpless continued, discussing the response of the National Cancer Institute to the COVID-19 pandemic in this recent period.
The NCI response is divided into three separate categories:
- Flexibility for grantees - including deadline extensions for applications and reports, funds carryover and other administrative flexibilities and extension of eligibility for early-stage investigators
- COVID-19 serology research - evaluation of commercially available COVID-19 serology tests on behalf of the Food and Drug Administration (FDA), and working to increase national capacity for high-quality serological testing
- Response in clinical trials - this includes flexibility for protocol deviations, informed consent made possible by phone, increased use of compassionate protocols, and implementation of the NCCAPS study
The NCCAPS study stands for the “NCI COVID-19 in cancer patients’ study” (NCT04387656). This is a longitudinal natural history study aiming to enroll 2000 or more cancer patients who were infected with COVID-19 at 1000 sites across the USA. It was launched on May 21st, 2020, and it took six weeks to implement this study from idea to launch. There has been a special emphasis on minority, underserved, and rural recruitment.
Additionally, there have also been several registries that have been formed specifically for cancer and COVID-19. These include the:
- COVID-19 and Cancer consortium (CCC19)
- ASH RC COVID-19 registry for hematologic malignancy
- ASCO registry
- Thoracic cancers international COVID-19 collaboration (TERAVOLT)
Dr. Sharpless Concluded his elegant talk sharing his concerns about the delay in cancer screening, diagnosis, slowing, and the reported higher cost in clinical trials and lab work in these troubling times. Despite these limitations, he emphasized how important it is to use all available resources in to overcome the hurdles that exist in an attempt to expedite all necessary steps of Cancer Research so that we can continue to improve the treatment of cancer patients, based on the state of the art evidence-based medicine.
Presented by: Norman E. Sharpless, MD, Director, National Cancer Institute, United States
Written By: Hanan Goldberg, MD, MSc., Urology Department, SUNY Upstate Medical University, Syracuse, NY, USA, Twitter: @GoldbergHanan, at the 2020 ASCO Annual Meeting, Virtual Scientific Program #ASCO20, May 29-31, 2020.


