Aarhus, Denmark (UroToday.com) Dr. Vikram Narayan from the University of Texas MD Anderson Cancer Center presented data on behalf of Dr. Justin Matulay and Dr. Neema Navai’s group summarizing a retrospective cohort study investigating the efficacy of non-cisplatin based neoadjuvant chemotherapy agents for cisplatin-ineligible patients prior to radical cystectomy.
Over 674 patients were included in the study with a median follow-up of 32.3 months, following a review of consecutive high-risk, clinically node-negative muscle-invasive bladder cancer (MIBC) patients undergoing radical cystectomy (RC) between 2005 and 2017 at the MD Anderson Cancer Center. Pre-operative high-risk criteria included one or more of the following: lymphovascular invasion, hydronephrosis, presence of extravesical disease, and/or variant histology. Primary outcomes were cancer-specific survival (CSS) and overall survival (OS). Cisplatin-based neoadjuvant chemotherapy (NAC) was used in 63% of patients, non-cisplatin NAC (defined as any regimen containing at least one cycle of cisplatin-therapy) was used in 11.6% of the cohort, while the remainder of patients (26%) proceeded to immediate radical cystectomy. Although patients who underwent chemotherapy had more high risk features than those who went straight to RC, administration of any NAC (either cisplatin-based or non-cisplatin based) was associated with a higher pathologic complete response rate (28.4% of cisNAC, 23.1% of non-cisNAC, and 7.5% of immediate RC).
Despite this, univariate OS and CSS improvements were only observed in patients receiving a cisplatin-containing regimen, and neither receipt of cisNAC nor non-cisNAC were independently predictive of survival outcomes. This finding may be secondary to unmeasured confounders but may also question the validity of continuing to use the pathologic complete response as a surrogate endpoint in clinical trials moving forward. Additionally, these data provide a benchmark for understanding outcomes of cisplatin-ineligible high-risk MIBC patients receiving alternative therapies.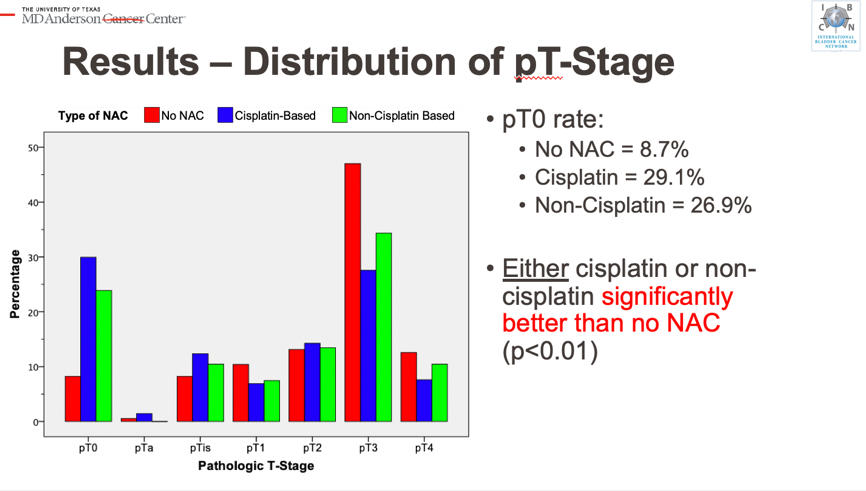
Abstract take-home message:
- Non-cisplatin neoadjuvant chemotherapy options for cisplatin-ineligible patients with an approximately 23% pathologic complete response rate at the time of radical cystectomy, although this did not correlate with improved survival outcomes.
Presented by: Vikram Narayan, Urologic Oncology Fellow, the University of Texas MD Anderson Cancer Center, Houston, Texas
Written by: Dr. Vikram M. Narayan (@VikramNarayan), Urologic Oncology Fellow with Ashish M. Kamat, MD (@UroDocAsh), Professor, Department of Urology, Division of Surgery, The University of Texas MD Anderson Cancer Center, Houston, TX at the 17th meeting of the International Bladder Cancer Network, (IBCN, #IBCN2019) October 3rd – 5th, 2019 in Aarhus, Denmark.


