Regarding study design, he first noted that the discussion of surgical options should have been discussed prior to randomization into either treatment arm as not doing so may have conferred some bias in the outcome. Second, he noted the overall duration of time required of four years to enroll the 120 patients on trial, reflective of difficulties enrolling in this trial space. Third, he noted the limitations of clinical T stage assessment, highlighting data from the PURE-01 trial (neoadjuvant pembrolizumab) that showed PET/CT-based staging identified an additional 7% of patients that were node-positive.
With regard to outcome assessment, Dr. Necchi emphasized the non-uniformity of assessment of clinical complete response, especially in patients who undergo organ preservation surgery. One possible way to overcome difficulties with assessing clinical CR is using multi-parametric MRI (mpMRI). Within the PURE-01 study, mpMRI had an AUC of ~0.75 for detecting pathologic complete response rates. Pending validation, Dr. Necchi argued that this tool may be a reasonable way to assess clinical CR. Additionally, Dr. Necchi noted that when comparing the cohorts within NEO-BLADE to the benchmarking neoadjuvant chemotherapy studies, the placebo (chemotherapy only) preformed quite poorly, which may have somewhat inflated the PFS and OS data. A larger prospective study would be required to more accurately assess this latter point and the overall truth of the statement that the addition of nintedanib offers a significant incremental advantage over the standard of care for neoadjuvant treatment of MIBC.
Dr. Necchi then discussed the BLASST-1 trial of nivolumab in combination with gemcitabine and cisplatin neoadjuvant therapy. He first pointed out that the patient cohort was heavily enriched for cT2 patients. He then commented on the primary endpoint of pathologic downgrading to non-muscle invasive disease at the time of surgery. The true pathological CR (pT0) was lower at 35% compared to the 66% of patients who had downstaging to non-invasive disease and cannot be interpreted as clearly beneficial over benchmarking neoadjuvant chemotherapy pCR rates.
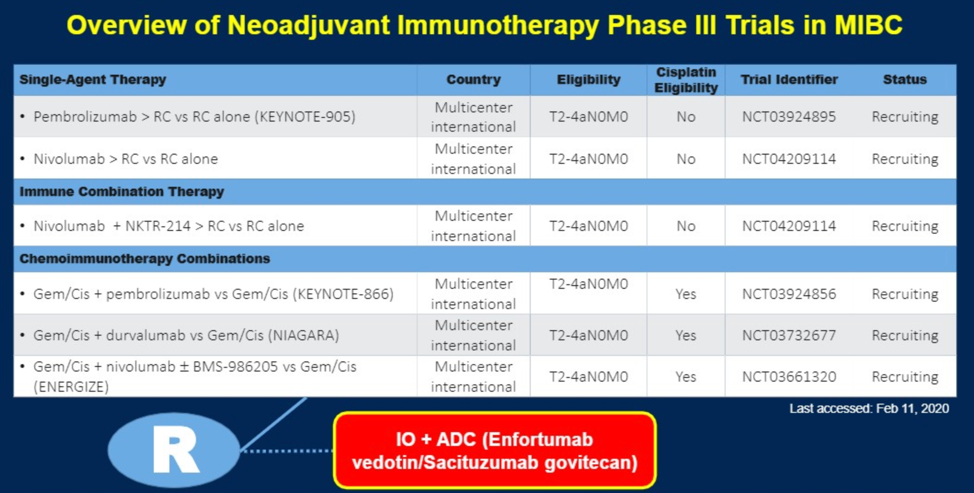
Dr. Necchi then reviewed the neoadjuvant immunotherapy phase 3 trials that are ongoing in MIBC, as shown below.
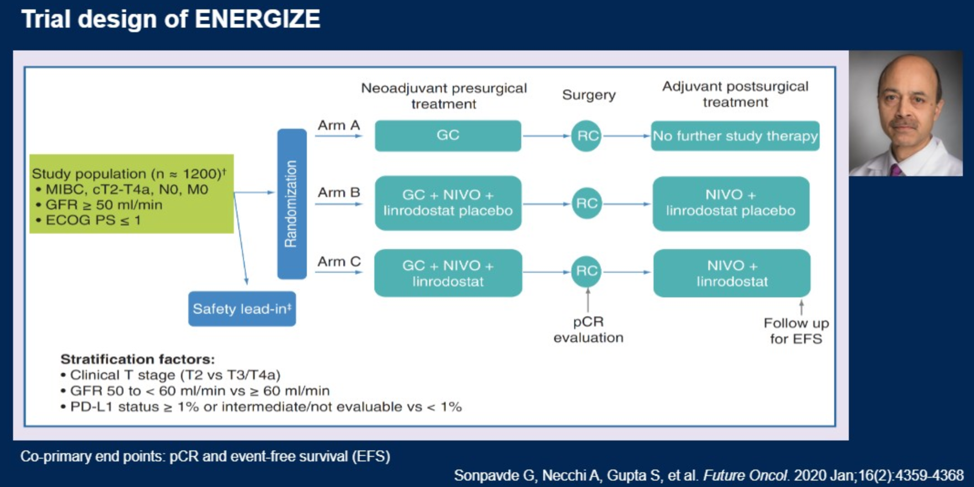
He specifically showed the design of the ENERGIZE trial, which is designed in part to further explore the role of nivolumab in neoadjuvant therapy.
He then discussed the importance of molecular subtyping for predicting response to neoadjuvant therapy.

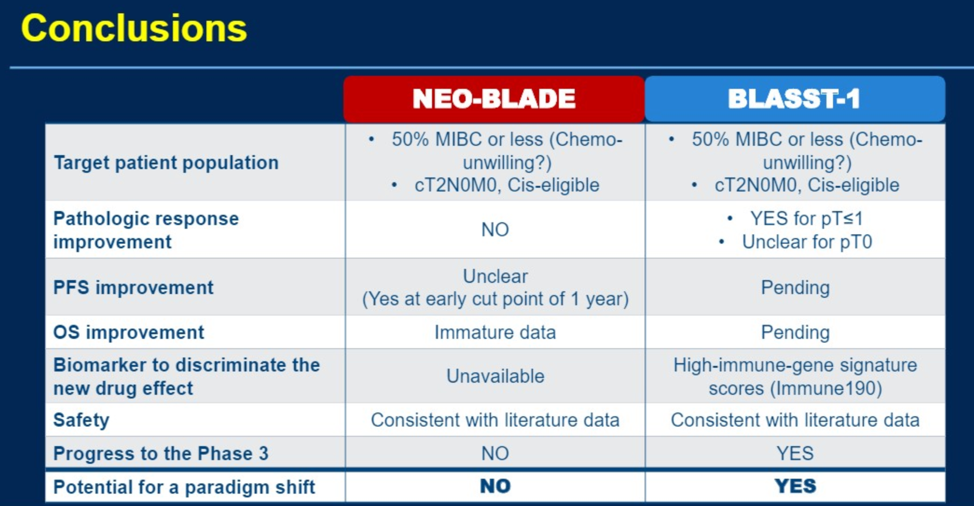
Finally, Dr. Necchi broke down his final assessment of the two abstracts by their clinical outcome and correlative data.
Presented by: Andrea Necchi, MD, MD | Department of Medical Oncology, Fondazione IRCCS Istituto Nazionale dei Tumori
Written by: Alok Tewari, MD, Ph.D., Medical Oncology Fellow at the Dana-Farber Cancer Institute, at the 2020 Genitourinary Cancers Symposium, ASCO GU #GU20, February 13-15, 2020, San Francisco, California
Related Content:
ASCO GU 2020: Results from BLASST-1 (Bladder Cancer Signal Seeking Trial) of Nivolumab, Gemcitabine, and Cisplatin in Muscle Invasive Bladder Cancer Undergoing Cystectomy
ASCO GU 2020: (NEO-BLADE) Phase II Randomized Placebo-Controlled Neoadjuvant Trial of Nintedanib or Placebo with Gemcitabine and Cisplatin in Locally Advanced Muscle Invasive Bladder Cancer