Strategies to enhance immunotherapeutic benefit in PC include:
1. Combination of immunotherapies (multiple vaccines, vaccine + immune checkpoint inhibitor [CPI], immunocytokine + CPI).
2. Keynote -046 is an example for a study combining a listeria based vaccine with Pembrolizumab in mCRPC patients. Another study assessed the combination of a PSA vaccine (prostvac) plus Nivolumab in mCRPC patients [2]
3. Combinations with therapies to capitalize on immunologic synergy. Such studies assessed the effect of addition of other accepted treatments such as enzalutamide, PARP inhibitors, Radium 223, and docetaxel, to immunotherapy. Docetaxel in fact, induces immunogenic modulations, and causes increased expression of ICAM-1, MUC-1, and MHC class 1 molecules [3].
4. Begin immunotherapy in early disease (castration sensitive). The rationale behind this is that there is only “micrometastatic disease” at this timepoint, which is associated with less immune suppression. Also, there is a different microenvironment in earlier disease.
IL-12 is an immunocytokine inducing differentiation of naïve CD4+ T-cells to the Th1 phenotype. It increases proliferation and lytic capacity of cytotoxic T lymphocyte (CTL) and natural killer (NK) cells. It also promotes IFN-y production by NK and T-cells. Systematic IL-12 is hampered by severe systemic toxicity. NHS-76 is an antibody that binds to DNA exposed in necrotic regions of tumors. The combination of NHS-IL12 was demonstrated to enhance anti-PDL1 activity.
M7824 is a bifunctional fusion protein composed of a monoclonal antibody directed against PD-L1, which is fused to the extracellular domain of human transforming growth factor–β (TGF-β) receptor II. It functions as a “trap” for all three TGF-β isoforms.
A future study plans to combine docetaxel with both NHS-IL12 and M7824 in 2 cohorts. Cohort 1 will include metastatic castrate sensitive PC patients that will be given 6 cycles of docetaxel+NHS-IL12+ M7824 and then continue to stage 2, to only receive NHS-IL12 and TGF-β. Cohort 2 will include patients with mCRPC who will receive the same treatment at first, but will not continue to stage 2. The end points will be progression free survival (PFS) and immunologic reactions.
Dr. Madan next discussed the strategy of starting immunotherapy early in the disease, in the stage of castration sensitive, when BCR has occurred, after potential curative salvage treatment has been given. At this stage, patients are asymptomatic and the available therapeutic options are either surveillance or ADT. Patients at this timepoint might only have micrometastatic disease, which is associated with less immune suppression. Furthermore, the testosterone levels are normal (as long as ADT is not being given) and it is not clear if this affects the immune system. The microenvironment is different at tis stage as well, and might be more lymph node based. Unfortunately, the available imaging today is very limited, restricting our ability to detect the full spectrum of disease. Emerging PET imaging strategies will enable us to fully see and diagnose the “real” disease at this stage.
Dr. Madan then described the PROSPECT study, where patients with BCR were randomized to either surveillance for 6 months and then given Prostvac vaccination, or upfront vaccination. This vaccine is injected subcutaneously, infects antigen presenting cells (APCs), which in turn activate T-cells, causing tumor destruction. The primary objective was to determine if this vaccination can decrease tumor growth as measured by PSA rise after 6 months, compared to a group on surveillance for 6 months (Figure 2).

The results demonstrated PSA fluctuations with eventual decline (Figure 3).

The preliminary conclusions for this study are that this vaccine may cause delayed PSA declines. This may provide some data that immunotherapy may be effective in this early stage of PC, as opposed to the mCRPC patients. It is unclear what these fluctuations of PSA levels mean on the long-term outcomes. Another similar planned trial, led by Dr. Doug McNeal, will involve a DNA based vaccine combined with Nivolumab in BCR patients. The primary objective will be safety/tolerability, and PSA complete response rate.
Dr. Madan concluded his interesting talk by reiterating some important points. Currently, immunotherapy has a limited role in PC. Future strategies may require altering the tumor microenvironment to make it more responsive to immune killing. Combinations with chemotherapy, radiotherapy, or other immunotherapy drugs/vaccines, may optimize in vivo immune activation. Lastly, earlier stages of PC, with less tumor burden, and normal testosterone levels, may have different responsiveness to immunotherapy compared to mCRPC.
Presented by: Ravi Madan, National Cancer Institute
Written by: Hanan Goldberg, MD, Urologic Oncology Fellow (SUO), University of Toronto, Princess Margaret Cancer Centre, Twitter:@GoldbergHanan at the 2018 ASCO Annual Meeting - June 1-5, 2018 – Chicago, IL USA
References:
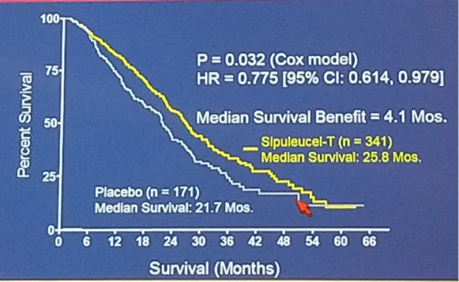
[1] Kantoff et al. NEJM 2010
[2] Madan RA et al. ASCO 2018
[3] Hodge JW. et al. Int. Journal of Cancer 2013


